Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte
- PMID: 23659999
- PMCID: PMC3699109
- DOI: 10.1242/jcs.128744
Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte
Abstract
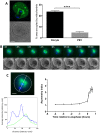
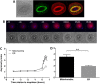
A fundamental rule of cell division is that daughter cells inherit half the DNA complement and an appropriate proportion of cellular organelles. The highly asymmetric cell divisions of female meiosis present a different challenge because one of the daughters, the polar body, is destined to degenerate, putting at risk essential maternally inherited organelles such as mitochondria. We have therefore investigated mitochondrial inheritance during the meiotic divisions of the mouse oocyte. We find that mitochondria are aggregated around the spindle by a dynein-mediated mechanism during meiosis I, and migrate together with the spindle towards the oocyte cortex. However, at cell division they are not equally segregated and move instead towards the oocyte-directed spindle pole and are excluded from the polar body. We show that this asymmetrical inheritance in favour of the oocyte is not caused by bias in the spindle itself but is dependent on an intact actin cytoskeleton, spindle-cortex proximity, and cell cycle progression. Thus, oocyte-biased inheritance of mitochondria is a variation on rules that normally govern organelle segregation at cell division, and ensures that essential maternally inherited mitochondria are retained to provide ATP for early mammalian development.
Keywords: Asymmetry; Mitochondria; Oocyte.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

