The use of the reinstatement model to study relapse to palatable food seeking during dieting
- PMID: 23660229
- PMCID: PMC3785569
- DOI: 10.1016/j.neuropharm.2013.04.030
The use of the reinstatement model to study relapse to palatable food seeking during dieting
Abstract
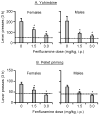
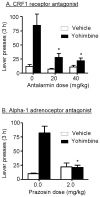
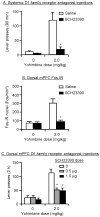
Excessive consumption of unhealthy foods is a major public health problem. While many people attempt to control their food intake through dieting, many relapse to unhealthy eating habits within a few months. We have begun to study this clinical condition in rats by adapting the reinstatement model, which has been used extensively to study relapse to drug seeking. In our adaptation of the relapse model, reinstatement of palatable food seeking by exposure to food-pellet priming, food-associated cues, or stress is assessed in food-restricted (to mimic dieting) rats after operant food-pellet self-administration training and subsequent extinction of the food-reinforced responding. In this review, we first outline the clinical problem and discuss a recent study in which we assessed the predictive validity of the reinstatement model for studying relapse to food seeking during dieting by using the anorexigenic drug fenfluramine. Next, we summarize results from our initial studies on the role of several stress- and feeding-related peptides (corticotropin-releasing factor, hypocretin, melanin-concentrating hormone, peptide YY3-36) in reinstatement of palatable food seeking. We then present results from our studies on the role of dopamine and medial prefrontal cortex in stress-induced reinstatement of food seeking. We conclude by discussing potential clinical implications. We offer two main conclusions: (1) the food reinstatement model is a simple, reliable, and valid model to study mechanisms of relapse to palatable food seeking during dieting, and to identify medications to prevent this relapse; (2) mechanisms of relapse to food seeking are often dissociable from mechanisms of ongoing food intake. This article is part of a Special Issue entitled 'NIDA 40th Anniversary Issue'.
Keywords: Dieting; Food; Priming; Reinstatement; Relapse; Self-administration; Stress; cue.
Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
Figures
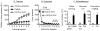
Similar articles
-
Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model.Psychopharmacology (Berl). 2012 May;221(2):341-53. doi: 10.1007/s00213-011-2585-9. Epub 2011 Dec 3. Psychopharmacology (Berl). 2012. PMID: 22134478 Free PMC article.
-
Peptide YY3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model.J Neurosci. 2007 Oct 24;27(43):11522-32. doi: 10.1523/JNEUROSCI.5405-06.2007. J Neurosci. 2007. PMID: 17959795 Free PMC article.
-
Effect of cafeteria diet history on cue-, pellet-priming-, and stress-induced reinstatement of food seeking in female rats.PLoS One. 2014 Jul 15;9(7):e102213. doi: 10.1371/journal.pone.0102213. eCollection 2014. PLoS One. 2014. PMID: 25025329 Free PMC article.
-
The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking.Prog Neurobiol. 2009 Sep;89(1):18-45. doi: 10.1016/j.pneurobio.2009.05.003. Epub 2009 Jun 2. Prog Neurobiol. 2009. PMID: 19497349 Free PMC article. Review.
-
The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research.Psychopharmacology (Berl). 2013 Oct;229(3):453-76. doi: 10.1007/s00213-013-3120-y. Epub 2013 May 18. Psychopharmacology (Berl). 2013. PMID: 23685858 Free PMC article. Review.
Cited by
-
Lorcaserin and CP-809101 reduce motor impulsivity and reinstatement of food seeking behavior in male rats: Implications for understanding the anti-obesity property of 5-HT2C receptor agonists.Psychopharmacology (Berl). 2016 Jul;233(14):2841-56. doi: 10.1007/s00213-016-4329-3. Epub 2016 May 30. Psychopharmacology (Berl). 2016. PMID: 27241709
-
Activation of peroxisome proliferator-activated receptor γ reduces alcohol drinking and seeking by modulating multiple mesocorticolimbic regions in rats.Neuropsychopharmacology. 2021 Jan;46(2):360-367. doi: 10.1038/s41386-020-0754-4. Epub 2020 Jul 1. Neuropsychopharmacology. 2021. PMID: 32610339 Free PMC article.
-
Hindbrain ghrelin and liver-expressed antimicrobial peptide 2, ligands for growth hormone secretagogue receptor, bidirectionally control food intake.Am J Physiol Regul Integr Comp Physiol. 2023 Apr 1;324(4):R547-R555. doi: 10.1152/ajpregu.00232.2022. Epub 2023 Feb 27. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 36847494 Free PMC article.
-
Vulnerability to diet-induced obesity is associated with greater food priming-induced reinstatement of palatable food seeking.Physiol Behav. 2020 Jan 1;213:112730. doi: 10.1016/j.physbeh.2019.112730. Epub 2019 Oct 31. Physiol Behav. 2020. PMID: 31678197 Free PMC article.
-
Role of Dorsomedial Striatum Neuronal Ensembles in Incubation of Methamphetamine Craving after Voluntary Abstinence.J Neurosci. 2017 Jan 25;37(4):1014-1027. doi: 10.1523/JNEUROSCI.3091-16.2016. J Neurosci. 2017. PMID: 28123032 Free PMC article.
References
-
- Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. - PubMed
-
- Adan RA. Mechanisms underlying current and future anti-obesity drugs. Trends Neurosci 2013 - PubMed
-
- Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. - PubMed
-
- Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

