Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats
- PMID: 23660230
- PMCID: PMC3769475
- DOI: 10.1016/j.neuropharm.2013.04.029
Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats
Abstract
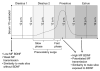
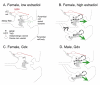
Many studies have described potent effects of BDNF, 17β-estradiol or androgen on hippocampal synapses and their plasticity. Far less information is available about the interactions between 17β-estradiol and BDNF in hippocampus, or interactions between androgen and BDNF in hippocampus. Here we review the regulation of BDNF in the mossy fiber pathway, a critical part of hippocampal circuitry. We discuss the emerging view that 17β-estradiol upregulates mossy fiber BDNF synthesis in the adult female rat, while testosterone exerts a tonic suppression of mossy fiber BDNF levels in the adult male rat. The consequences are interesting to consider: in females, increased excitability associated with high levels of BDNF in mossy fibers could improve normal functions of area CA3, such as the ability to perform pattern completion. However, memory retrieval may lead to anxiety if stressful events are recalled. Therefore, the actions of 17β-estradiol on the mossy fiber pathway in females may provide a potential explanation for the greater incidence of anxiety-related disorders and post-traumatic stress syndrome (PTSD) in women relative to men. In males, suppression of BDNF-dependent plasticity in the mossy fibers may be protective, but at the 'price' of reduced synaptic plasticity in CA3. This article is part of the Special Issue entitled 'BDNF Regulation of Synaptic Structure, Function, and Plasticity'.
Keywords: Area CA3; Estradiol; Hippocampus; Mossy fiber sprouting; Neurotrophin; Testosterone.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
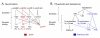





Similar articles
-
At immature mossy-fiber-CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA.J Neurosci. 2009 Feb 25;29(8):2637-47. doi: 10.1523/JNEUROSCI.5019-08.2009. J Neurosci. 2009. PMID: 19244539 Free PMC article.
-
In vivo BDNF modulation of hippocampal mossy fiber plasticity induced by high frequency stimulation.Hippocampus. 2012 Jan;22(1):1-8. doi: 10.1002/hipo.20866. Epub 2010 Sep 16. Hippocampus. 2012. PMID: 20848610
-
Enhanced mossy fiber sprouting and synapse formation in organotypic hippocampal cultures following transient domoic acid excitotoxicity.Neurotox Res. 2014 May;25(4):402-10. doi: 10.1007/s12640-013-9450-z. Epub 2013 Dec 18. Neurotox Res. 2014. PMID: 24347374
-
Androgen Modulation of Hippocampal Structure and Function.Neuroscientist. 2016 Feb;22(1):46-60. doi: 10.1177/1073858414558065. Epub 2014 Nov 21. Neuroscientist. 2016. PMID: 25416742 Free PMC article. Review.
-
Revisiting the role of the hippocampal mossy fiber synapse.Hippocampus. 2001;11(4):408-17. doi: 10.1002/hipo.1055. Hippocampus. 2001. PMID: 11530845 Review.
Cited by
-
A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release.J Cell Biol. 2015 Sep 28;210(7):1225-37. doi: 10.1083/jcb.201504092. Epub 2015 Sep 21. J Cell Biol. 2015. PMID: 26391661 Free PMC article.
-
Regulation of Expression of Hyperalgesic Priming by Estrogen Receptor α in the Rat.J Pain. 2017 May;18(5):574-582. doi: 10.1016/j.jpain.2016.12.017. Epub 2017 Jan 9. J Pain. 2017. PMID: 28089711 Free PMC article.
-
Sex differences in hippocampal area CA3 pyramidal cells.J Neurosci Res. 2017 Jan 2;95(1-2):563-575. doi: 10.1002/jnr.23927. J Neurosci Res. 2017. PMID: 27870399 Free PMC article. Review.
-
Sex Hormones, BDNF, Leptin, and TGF-β1 in Females With IBS: A Pilot Investigation.Biol Res Nurs. 2021 Apr;23(2):231-237. doi: 10.1177/1099800420948589. Epub 2020 Aug 18. Biol Res Nurs. 2021. PMID: 32806924 Free PMC article.
-
Opioid receptor-dependent sex differences in synaptic plasticity in the hippocampal mossy fiber pathway of the adult rat.J Neurosci. 2015 Jan 28;35(4):1723-38. doi: 10.1523/JNEUROSCI.0820-14.2015. J Neurosci. 2015. PMID: 25632146 Free PMC article.
References
-
- Altemus M, Epstein L. Sex differences in anxiety disorders. In: Becker JB, Berkley KJ, Feary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain. Oxford University Press; Oxford, U.K.: 2008. pp. 397–397.
-
- Barde YA, Davies AM, Johnson JE, Lindsay RM, Thoenen H. Brain derived neurotrophic factor. Prog Brain Res. 1987;71:185–189. - PubMed
-
- Bergquist F, Ludvig M. Neuropeptide release. In: Malenka RC, editor. Intercellular communication in the nervous system. Academic Press; New York: 2009. pp. 519–519.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

