Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug
- PMID: 23660251
- PMCID: PMC3901568
- DOI: 10.1016/j.biomaterials.2013.04.016
Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug
Abstract
Immuno-isolation of islets has the potential to enable the replacement of pancreatic function in diabetic patients. However, host response to the encapsulated islets frequently leads to fibrotic overgrowth with subsequent impairment of the transplanted grafts. Here, we identified and incorporated anti-inflammatory agents into islet-containing microcapsules to address this challenge. In vivo subcutaneous screening of 16 small molecule anti-inflammatory drugs was performed to identify promising compounds that could minimize the formation of fibrotic cell layers. Using parallel non-invasive fluorescent and bioluminescent imaging, we identified dexamethasone and curcumin as the most effective drugs in inhibiting the activities of inflammatory proteases and reactive oxygen species in the host response to subcutaneously injected biomaterials. Next, we demonstrated that co-encapsulating curcumin with pancreatic rat islets in alginate microcapsules reduced fibrotic overgrowth and improved glycemic control in a mouse model of chemically-induced type I diabetes. These results showed that localized administration of anti-inflammatory drug can improve the longevity of encapsulated islets and may facilitate the translation of this technology toward a long-term cure for type I diabetes.
Published by Elsevier Ltd.
Conflict of interest statement
The authors declared no conflict of interest.
Figures

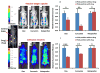
 ) and with drugs (
) and with drugs (
 ) B–D) Bioluminescent images of representative mice on day 2 at the peak of ROS activity. E) Quantified bioluminescent signals on day 2. F–H) Fluorescent images of representative mice on day 9 at the peak of cathepsin activity. I) Quantified fluorescent signals on day 9. Data: mean ± s.e.m (n=15 replicate injections). *, **, *** denotes p<0.05, 0.01, 0.0001 respectively.
) B–D) Bioluminescent images of representative mice on day 2 at the peak of ROS activity. E) Quantified bioluminescent signals on day 2. F–H) Fluorescent images of representative mice on day 9 at the peak of cathepsin activity. I) Quantified fluorescent signals on day 9. Data: mean ± s.e.m (n=15 replicate injections). *, **, *** denotes p<0.05, 0.01, 0.0001 respectively.


Similar articles
-
Construction of functional pancreatic artificial islet tissue composed of fibroblast-modified polylactic- co-glycolic acid membrane and pancreatic stem cells.J Biomater Appl. 2017 Sep;32(3):362-372. doi: 10.1177/0885328217722041. Epub 2017 Jul 26. J Biomater Appl. 2017. PMID: 28747082
-
In vivo vascularization of anisotropic channeled porous polylactide-based capsules for islet transplantation: the effects of scaffold architecture and implantation site.Physiol Res. 2015;64(Suppl 1):S75-84. doi: 10.33549/physiolres.933138. Physiol Res. 2015. PMID: 26447597
-
Improvement of islet function and survival by integration of perfluorodecalin into microcapsules in vivo and in vitro.J Tissue Eng Regen Med. 2018 Apr;12(4):e2110-e2122. doi: 10.1002/term.2643. Epub 2018 Feb 27. J Tissue Eng Regen Med. 2018. PMID: 29330944
-
Factors influencing the properties and performance of microcapsules for immunoprotection of pancreatic islets.J Mol Med (Berl). 1999 Jan;77(1):199-205. doi: 10.1007/s001090050336. J Mol Med (Berl). 1999. PMID: 9930963 Review.
-
Encapsulated Islet Transplantation: Where Do We Stand?Rev Diabet Stud. 2017 Spring;14(1):51-78. doi: 10.1900/RDS.2017.14.51. Epub 2017 Jun 12. Rev Diabet Stud. 2017. PMID: 28632821 Free PMC article. Review.
Cited by
-
A Closed-Loop Autologous Erythrocyte-Mediated Delivery Platform for Diabetic Nephropathy Therapy.Nanomaterials (Basel). 2022 Oct 11;12(20):3556. doi: 10.3390/nano12203556. Nanomaterials (Basel). 2022. PMID: 36296745 Free PMC article.
-
Electrospraying an enabling technology for pharmaceutical and biomedical applications: A review.J Aerosol Sci. 2018 Nov;125:164-181. doi: 10.1016/j.jaerosci.2018.04.002. Epub 2018 Apr 9. J Aerosol Sci. 2018. PMID: 30662086 Free PMC article.
-
Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications.Mater Today Bio. 2021 Dec 9;13:100186. doi: 10.1016/j.mtbio.2021.100186. eCollection 2022 Jan. Mater Today Bio. 2021. PMID: 34917924 Free PMC article. Review.
-
Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates.Nat Biotechnol. 2016 Mar;34(3):345-52. doi: 10.1038/nbt.3462. Epub 2016 Jan 25. Nat Biotechnol. 2016. PMID: 26807527 Free PMC article.
-
Hydrogel-Encapsulated Pancreatic Islet Cells as a Promising Strategy for Diabetic Cell Therapy.Research (Wash D C). 2024 Jul 4;7:0403. doi: 10.34133/research.0403. eCollection 2024. Research (Wash D C). 2024. PMID: 38966749 Free PMC article. Review.
References
-
- Kuhtreiber WM, Lanza RP, Chick WL. Cell encapsulation technology and therapeutics. Boston: Birkhauser; 1999.
-
- Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–10. - PubMed
-
- Calafiore R, Basta G, Luca G, Lemmi A, Montanucci MP, Calabrese G, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes. Diabetes Care. 2006;29(1):137–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

