Integration of satiety signals by the central nervous system
- PMID: 23660361
- PMCID: PMC3688053
- DOI: 10.1016/j.cub.2013.03.020
Integration of satiety signals by the central nervous system
Abstract
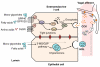
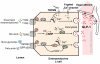
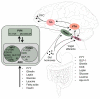
Individual meals are products of a complex interaction of signals related to both short-term and long-term availability of energy stores. In addition to maintaining the metabolic demands of the individual in the short term, levels of energy intake must also maintain and defend body weight over longer periods. To accomplish this, satiety pathways are regulated by a sophisticated network of endocrine and neuroendocrine pathways. Higher brain centers modulate meal size through descending inputs to caudal brainstem regions responsible for the motor pattern generators associated with ingestion. Gastric and intestinal signals interact with central nervous system pathways to terminate food intake. These inputs can be modified as a function of internal metabolic signals, external environmental influences, and learning to regulate meal size.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
References
-
- Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. - PubMed
-
- Langhans W, Geary N. Overview of the physiological control of eating. Forum Nutr. 2009;63:9–53. - PubMed
-
- Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. American Journal of Physiology. 1996;271:R766–R769. - PubMed
-
- Kaplan JM, Moran TH. Gastrointestinal signaling in the control of food intake. In: Stricker EM, Woods SC, editors. Handbook of Behavioral Neurobiology. Neurobiology of Food and Fluid Intake. no. 2. vol. 4. Kluwer Academic/Plenum Publishing; New York: 2004. pp. 273–303.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

