The myelodysplastic syndrome as a prototypical epigenetic disease
- PMID: 23660859
- PMCID: PMC3650703
- DOI: 10.1182/blood-2013-02-451757
The myelodysplastic syndrome as a prototypical epigenetic disease
Abstract
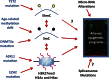
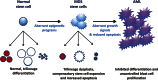
The myelodysplastic syndrome (MDS) is a clonal disorder characterized by increased stem cell proliferation coupled with aberrant differentiation resulting in a high rate of apoptosis and eventual symptoms related to bone marrow failure. Cellular differentiation is an epigenetic process that requires specific and highly ordered DNA methylation and histone modification programs. Aberrant differentiation in MDS can often be traced to abnormal DNA methylation (both gains and losses of DNA methylation genome wide and at specific loci) as well as mutations in genes that regulate epigenetic programs (TET2 and DNMT3a, both involved in DNA methylation control; EZH2 and ASXL1, both involved in histone methylation control). The epigenetic nature of MDS may explain in part the serendipitous observation that it is the disease most responsive to DNA methylation inhibitors; other epigenetic-acting drugs are being explored in MDS as well. Progression in MDS is characterized by further acquisition of epigenetic defects as well as mutations in growth-controlling genes that seem to tip the proliferation/apoptosis balance and result in the development of acute myelogenous leukemia. Although MDS is clinically and physiologically heterogeneous, a case can be made that subsets of the disease can be largely explained by disordered stem cell epigenetics.
Figures
References
-
- Komrokji RS, Zhang L, Bennett JM. Myelodysplastic syndromes classification and risk stratification. Hematol Oncol Clin North Am. 2010;24(2):443–457. - PubMed
-
- Foran JM, Shammo JM. Clinical presentation, diagnosis, and prognosis of myelodysplastic syndromes. Am J Med. 2012;125(suppl 7):S6–S13. - PubMed
-
- Richert-Boe KE, Bagby GC., Jr In vitro hematopoiesis in myelodysplasia: liquid and soft-gel culture studies. Hematol Oncol Clin North Am. 1992;6(3):543–556. - PubMed
-
- Theilgaard-Mönch K, Boultwood J, Ferrari S, et al. Gene expression profiling in MDS and AML: potential and future avenues. Leukemia. 2011;25(6):909–920. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

