FoxO3a is activated and executes neuron death via Bim in response to β-amyloid
- PMID: 23661003
- PMCID: PMC3674357
- DOI: 10.1038/cddis.2013.148
FoxO3a is activated and executes neuron death via Bim in response to β-amyloid
Abstract
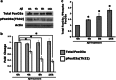
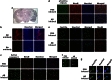
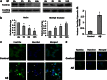
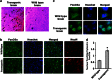
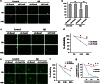
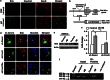
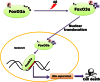
The molecules that mediate death of selective neurons in Alzheimer's disease (AD) are mostly unknown. The Forkhead transcription factor FoxO3a has emerged as an important mediator of cell fate including apoptosis. When phosphorylated by Akt, it is localized in the cytosol as an inactive complex bound with 14-3-3 protein. For activation and localization of FoxO3a in the nucleus, further modifications are required, such as phosphorylation by mammalian sterile 20-like kinase 1 (MST1) and arginine methylation by protein arginine methyltransferase1. We report here that Akt-mediated phosphorylation of FoxO3a is diminished in neurons exposed to oligomeric β-amyloid (Aβ), in vitro and in vivo. We also find that oligomeric Aβ activates FoxO3a by MST1 phosphorylation and arginine methylation in primary cultures of hippocampal and cortical neurons. Moreover, FoxO3a translocates from the cytosol to nucleus in cultured neurons in response to Aβ. Most importantly, the nuclear redistribution of FoxO3a is significantly increased in Aβ-overexpressing AβPPswe-PS1dE9 mice and Aβ-infused rat brains. We further find that FoxO3a is essential for loss of neurons and neural networks in response to Aβ. Recent reports implicate Bim, a pro-apoptotic member of Bcl-2 family, in neuron death in AD, as a key target of this transcription factor. We show that Bim is a direct target of FoxO3a in Aβ-treated neurons. Our findings thus indicate that FoxO3a is activated, translocated to the nucleus and mediates neuron death via Bim in response to Aβ toxicity.
Figures

References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

