Compartmentalization of GABAergic inhibition by dendritic spines
- PMID: 23661763
- PMCID: PMC3752161
- DOI: 10.1126/science.1234274
Compartmentalization of GABAergic inhibition by dendritic spines
Abstract
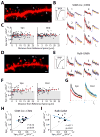
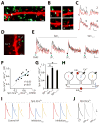
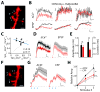
γ-aminobutyric acid-mediated (GABAergic) inhibition plays a critical role in shaping neuronal activity in the neocortex. Numerous experimental investigations have examined perisomatic inhibitory synapses, which control action potential output from pyramidal neurons. However, most inhibitory synapses in the neocortex are formed onto pyramidal cell dendrites, where theoretical studies suggest they may focally regulate cellular activity. The precision of GABAergic control over dendritic electrical and biochemical signaling is unknown. By using cell type-specific optical stimulation in combination with two-photon calcium (Ca(2+)) imaging, we show that somatostatin-expressing interneurons exert compartmentalized control over postsynaptic Ca(2+) signals within individual dendritic spines. This highly focal inhibitory action is mediated by a subset of GABAergic synapses that directly target spine heads. GABAergic inhibition thus participates in localized control of dendritic electrical and biochemical signaling.
Figures

Comment in
-
Synaptic transmission: keeping calcium contained.Nat Rev Neurosci. 2013 Jul;14(7):456. doi: 10.1038/nrn3527. Epub 2013 May 30. Nat Rev Neurosci. 2013. PMID: 23719570 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

