Scaffolding proteins DLG1 and CASK cooperate to maintain the nephron progenitor population during kidney development
- PMID: 23661808
- PMCID: PMC3699830
- DOI: 10.1681/ASN.2012111074
Scaffolding proteins DLG1 and CASK cooperate to maintain the nephron progenitor population during kidney development
Abstract
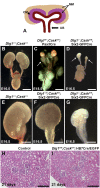
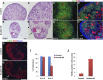
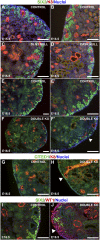
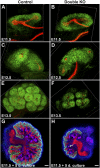
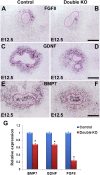
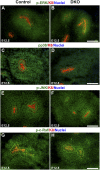
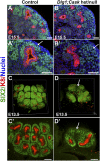
DLG1 (discs-large homolog 1) and CASK (calcium/calmodulin-dependent serine protein kinase) interact at membrane-cytoskeleton interfaces and function as scaffolding proteins that link signaling molecules, receptors, and other scaffolding proteins at intercellular and synaptic junctions. Dlg1-null mice exhibit hydronephrosis, hydroureter, and occasionally hypoplastic kidneys, whereas Cask-null mice do not. To investigate whether DLG1 and CASK cooperate in the developing urogenital system, we generated mice deficient in both DLG1 and CASK either 1) globally, 2) in metanephric mesenchyme, or 3) in nephron progenitors. With each approach, Dlg1;Cask double-knockout (DKO) kidneys were severely hypoplastic and dysplastic and demonstrated rapid, premature depletion of nephron progenitors/stem cells. Several cellular and molecular defects were observed in the DKO kidneys, including reduced proliferation and increased apoptosis of cells in the nephrogenic zone and a progressive decrease in the number of cells expressing SIX2, a transcription factor essential for maintaining nephron progenitors. Fgf8 expression was reduced in early-stage DKO metanephric mesenchyme, accompanied by reduced levels of components of the Ras pathway, which is activated by fibroblast growth factor (FGF) signaling. Moreover, Dlg1(+/-);Cask(-/-) (het/null) kidneys were moderately hypoplastic and demonstrated impaired aggregation of SIX2-positive cells around the ureteric bud tips. Nephron progenitor-specific het/null mice survived with small kidneys but developed glomerulocystic kidney disease and renal failure. Taken together, these results suggest that DLG1 and CASK play critical cooperative roles in maintaining the nephron progenitor population, potentially via a mechanism involving effects on FGF signaling.
Figures


Similar articles
-
DLG1 influences distal ureter maturation via a non-epithelial cell autonomous mechanism involving reduced retinoic acid signaling, Ret expression, and apoptosis.Dev Biol. 2014 Jun 15;390(2):160-9. doi: 10.1016/j.ydbio.2014.03.014. Epub 2014 Mar 31. Dev Biol. 2014. PMID: 24699546 Free PMC article.
-
Mdm2 is required for maintenance of the nephrogenic niche.Dev Biol. 2014 Mar 1;387(1):1-14. doi: 10.1016/j.ydbio.2014.01.009. Epub 2014 Jan 17. Dev Biol. 2014. PMID: 24440154 Free PMC article.
-
Mutagenesis of the epithelial polarity gene, discs large 1, perturbs nephrogenesis in the developing mouse kidney.Kidney Int. 2005 Sep;68(3):955-65. doi: 10.1111/j.1523-1755.2005.00489.x. Kidney Int. 2005. PMID: 16105026
-
Defining and redefining the nephron progenitor population.Pediatr Nephrol. 2011 Sep;26(9):1395-406. doi: 10.1007/s00467-010-1750-4. Epub 2011 Jan 14. Pediatr Nephrol. 2011. PMID: 21229268 Free PMC article. Review.
-
Nephron progenitors in the metanephric mesenchyme.Pediatr Nephrol. 2011 Sep;26(9):1463-7. doi: 10.1007/s00467-011-1806-0. Epub 2011 Feb 19. Pediatr Nephrol. 2011. PMID: 21336811 Review.
Cited by
-
CASK loss of function differentially regulates neuronal maturation and synaptic function in human induced cortical excitatory neurons.iScience. 2022 Sep 23;25(10):105187. doi: 10.1016/j.isci.2022.105187. eCollection 2022 Oct 21. iScience. 2022. PMID: 36262316 Free PMC article.
-
Fibroblast growth factor receptor signaling in kidney and lower urinary tract development.Pediatr Nephrol. 2016 Jun;31(6):885-95. doi: 10.1007/s00467-015-3151-1. Epub 2015 Aug 21. Pediatr Nephrol. 2016. PMID: 26293980 Free PMC article. Review.
-
Renal progenitors and childhood: from development to disorders.Pediatr Nephrol. 2014 Apr;29(4):711-9. doi: 10.1007/s00467-013-2686-2. Epub 2014 Jan 4. Pediatr Nephrol. 2014. PMID: 24389601 Review.
-
MAPK/ERK Signaling in Regulation of Renal Differentiation.Int J Mol Sci. 2019 Apr 10;20(7):1779. doi: 10.3390/ijms20071779. Int J Mol Sci. 2019. PMID: 30974877 Free PMC article. Review.
-
DLG1 functions upstream of SDCCAG3 and IFT20 to control ciliary targeting of polycystin-2.EMBO Rep. 2024 Jul;25(7):3040-3063. doi: 10.1038/s44319-024-00170-1. Epub 2024 Jun 7. EMBO Rep. 2024. PMID: 38849673 Free PMC article.
References
-
- Saxén L, Sariola H: Early organogenesis of the kidney. Pediatr Nephrol 1: 385–392, 1987 - PubMed
-
- Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 - PubMed
-
- Vainio S, Lin Y: Coordinating early kidney development: Lessons from gene targeting. Nat Rev Genet 3: 533–543, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

