Analysis of the stabilized supralinear network
- PMID: 23663149
- PMCID: PMC4026108
- DOI: 10.1162/NECO_a_00472
Analysis of the stabilized supralinear network
Abstract
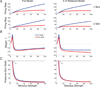
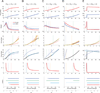
We study a rate-model neural network composed of excitatory and inhibitory neurons in which neuronal input-output functions are power laws with a power greater than 1, as observed in primary visual cortex. This supralinear input-output function leads to supralinear summation of network responses to multiple inputs for weak inputs. We show that for stronger inputs, which would drive the excitatory subnetwork to instability, the network will dynamically stabilize provided feedback inhibition is sufficiently strong. For a wide range of network and stimulus parameters, this dynamic stabilization yields a transition from supralinear to sublinear summation of network responses to multiple inputs. We compare this to the dynamic stabilization in the balanced network, which yields only linear behavior. We more exhaustively analyze the two-dimensional case of one excitatory and one inhibitory population. We show that in this case, dynamic stabilization will occur whenever the determinant of the weight matrix is positive and the inhibitory time constant is sufficiently small, and analyze the conditions for supersaturation, or decrease of firing rates with increasing stimulus contrast (which represents increasing input firing rates). In work to be presented elsewhere, we have found that this transition from supralinear to sublinear summation can explain a wide variety of nonlinearities in cerebral cortical processing.
Figures


References
-
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84(2):909–926. - PubMed
-
- Anderson JS, Lampl I, Gillespie D, Ferster D. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science. 2000;290:1968–1972. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

