Methods to increase participation in organised screening programs: a systematic review
- PMID: 23663511
- PMCID: PMC3686655
- DOI: 10.1186/1471-2458-13-464
Methods to increase participation in organised screening programs: a systematic review
Abstract
Background: The European Community recommends the implementation of population-based screening programmes for cervical, breast, and colorectal cancers. This recommendation is supported by many observational studies showing that organised programmes effectively reduce mortality and control the inappropriate use of screening tests. We conducted a systematic review of studies assessing the efficacy of interventions to increase participation in organised population-based screening programs.
Methods: We included all studies on interventions aimed at increasing screening participation published between 1/1999 and 7/2012. For those published before 1999, we considered the Jepson et al. (2000) review (Health Technol Assess 4:1-133, 2000).
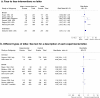
Results: Including studies from the Jepson review, we found 69 with quantitative information on interventions in organised screening: 19 for cervical, 26 for breast, 20 colorectal cancers, and 4 for cervical and breast cancer together.Effective interventions were: postal (breast RR = 1,37 95% Confidence Interval (95% CI): 1.25-1.51; cervical RR = 1.71 95% CI: 1.60-1.83; colorectal RR = 1.33 95% CI: 1.17-1.51) and telephone reminders (with heterogeneous methods for implementation); GP's signature on invitation letter (breast RR = 1.13 95% CI: 1.11-1.16; cervical RR = 1.20 95% CI: 1.10-1.30; colorectal RR = 1.15 95% CI: 1.07-1.24); scheduled appointment instead of open appointment (breast RR = 1.26 95% CI: 1.02-1.55; cervical RR = 1.49 95% CI: 1.27-1.75; colorectal RR = 1.79 95% CI: 1.65-1.93). Mailing a kit for self-sampling cervical specimens increased participation in non-responders (RR = 2.37 95% CI: 1.44-3.90).
Conclusion: Although some interventions did prove to be effective, some specific variables may influence their effectiveness in and applicability to organised population-based screening programs.
Figures







References
-
- European Council. Council Recommendation 2 December 2003 on cancer screening. Off J Eur Union. 2003. 2003/878/EC.
-
- Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, Wiener H, Herbert A, von Karsa L. In: European Guidelines for Quality Assurance in Cervical Cancer Screening. 2. European Commission, editor. Luxembourg: Office for Official Publications of the European Communities; 2008. pp. 1–291.
-
- IARC. Breast cancer screening. IARC Hanbooks of Cancer Prevention, Volume 7. Lyon: IARC Press; 2002.
-
- Segnan N, Patnick J, Karsa L von, editor. European guidelines for quality assurance in colorectal cancer screening and diagnosis. Luxembourg: European Commission; 2010. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

