Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy
- PMID: 23663781
- PMCID: PMC3677132
- DOI: 10.1016/j.cell.2013.04.015
Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy
Abstract
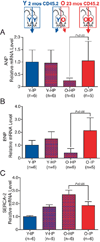
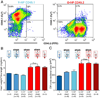
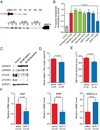
The most common form of heart failure occurs with normal systolic function and often involves cardiac hypertrophy in the elderly. To clarify the biological mechanisms that drive cardiac hypertrophy in aging, we tested the influence of circulating factors using heterochronic parabiosis, a surgical technique in which joining of animals of different ages leads to a shared circulation. After 4 weeks of exposure to the circulation of young mice, cardiac hypertrophy in old mice dramatically regressed, accompanied by reduced cardiomyocyte size and molecular remodeling. Reversal of age-related hypertrophy was not attributable to hemodynamic or behavioral effects of parabiosis, implicating a blood-borne factor. Using modified aptamer-based proteomics, we identified the TGF-β superfamily member GDF11 as a circulating factor in young mice that declines with age. Treatment of old mice to restore GDF11 to youthful levels recapitulated the effects of parabiosis and reversed age-related hypertrophy, revealing a therapeutic opportunity for cardiac aging.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
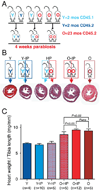
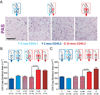
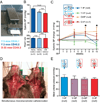
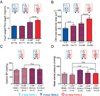
Comment in
-
Young at heart.Cell. 2013 May 9;153(4):743-5. doi: 10.1016/j.cell.2013.04.038. Cell. 2013. PMID: 23663775
-
Cardiovascular disease: Rejuvenating the ageing heart.Nat Rev Drug Discov. 2013 Jul;12(7):503. doi: 10.1038/nrd4064. Nat Rev Drug Discov. 2013. PMID: 23812265 No abstract available.
-
Ageing of the heart reversed by youthful systemic factors!EMBO J. 2013 Aug 14;32(16):2189-90. doi: 10.1038/emboj.2013.162. Epub 2013 Jul 16. EMBO J. 2013. PMID: 23860129 Free PMC article.
-
Cardiac aging and rejuvenation--a sense of humors?N Engl J Med. 2013 Aug 8;369(6):575-6. doi: 10.1056/NEJMcibr1306063. N Engl J Med. 2013. PMID: 23924010 No abstract available.
-
Through thick and thin: a circulating growth factor inhibits age-related cardiac hypertrophy.Circ Res. 2013 Aug 16;113(5):487-91. doi: 10.1161/CIRCRESAHA.113.302239. Circ Res. 2013. PMID: 23948581 Free PMC article.
-
Identification of a growth factor that rejuvenates the heart.Circ Cardiovasc Genet. 2013 Aug;6(4):435-6. doi: 10.1161/CIRCGENETICS.113.000274. Circ Cardiovasc Genet. 2013. PMID: 23963162 No abstract available.
References
-
- Aurigemma GP. Diastolic heart failure--a common and lethal condition by any name. N Engl J Med. 2006;355:308–310. - PubMed
-
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. - PubMed
-
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. - PubMed
-
- Bunster E, Meyer RK. An improved method of parabiosis. The Anatomical Record. 1933;57:339–343.
Publication types
MeSH terms
Substances
Grants and funding
- 5U01 HL100402/HL/NHLBI NIH HHS/United States
- 1R01 AG033053/AG/NIA NIH HHS/United States
- K99 HL112905/HL/NHLBI NIH HHS/United States
- 1R01 AG040019/AG/NIA NIH HHS/United States
- DP2 OD004345/OD/NIH HHS/United States
- R00 HL112905/HL/NHLBI NIH HHS/United States
- 1DP2 OD004345/OD/NIH HHS/United States
- K08 DK090147/DK/NIDDK NIH HHS/United States
- P30DK036836/DK/NIDDK NIH HHS/United States
- R01 AG033053/AG/NIA NIH HHS/United States
- R01 AG032977/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AG040019/AG/NIA NIH HHS/United States
- U01 HL100402/HL/NHLBI NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

