Mitochondrial localization and the persistent migration of epithelial cancer cells
- PMID: 23663851
- PMCID: PMC3647149
- DOI: 10.1016/j.bpj.2013.03.025
Mitochondrial localization and the persistent migration of epithelial cancer cells
Abstract
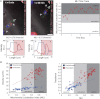
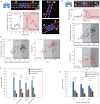
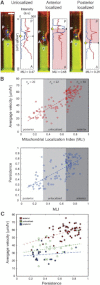
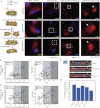
During cancer cell invasion, faster moving cancer cells play a dominant role by invading further and metastasizing earlier. Despite the importance of these outlier cells, the source of heterogeneity in their migratory behavior remains poorly understood. Here, we show that anterior localization of mitochondria, in between the nucleus and the leading edge of migrating epithelial cancer cells, correlates with faster migration velocities and increased directional persistence. The asymmetry of mitochondrial localization along the axis of migration is absent during spontaneous cell migration on two-dimensional surfaces and only occurs in the presence of chemical attractant cues or in conditions of mechanical confinement. Moreover, perturbing the asymmetric distribution of mitochondria within migrating cells by interfering with mitochondrial fusion (opa-1) or fission (drp-1) proteins, significantly reduces the number of cells with anterior localization of mitochondria and significantly decreases the velocity and directional persistence of the fastest moving cells. We also observed similar changes after perturbing the linkage between mitochondria and microtubules by the knockdown of mitochondrial rhoGTPase-1 (miro-1). Taken together, the changes in migration velocity and directional persistence in cells with anterior-localized mitochondria could account for an order of magnitude differences in invasive abilities between cells from otherwise homogenous cell populations.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Ridley A.J., Schwartz M.A., Horwitz A.R. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. - PubMed
-
- Pouthas F., Girard P., Reynaud E.G. In migrating cells, the Golgi complex and the position of the centrosome depend on geometrical constraints of the substratum. J. Cell Sci. 2008;121:2406–2414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

