Defective initiation of glycosaminoglycan synthesis due to B3GALT6 mutations causes a pleiotropic Ehlers-Danlos-syndrome-like connective tissue disorder
- PMID: 23664118
- PMCID: PMC3675258
- DOI: 10.1016/j.ajhg.2013.04.016
Defective initiation of glycosaminoglycan synthesis due to B3GALT6 mutations causes a pleiotropic Ehlers-Danlos-syndrome-like connective tissue disorder
Abstract
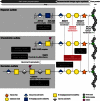
Proteoglycans are important components of cell plasma membranes and extracellular matrices of connective tissues. They consist of glycosaminoglycan chains attached to a core protein via a tetrasaccharide linkage, whereby the addition of the third residue is catalyzed by galactosyltransferase II (β3GalT6), encoded by B3GALT6. Homozygosity mapping and candidate gene sequence analysis in three independent families, presenting a severe autosomal-recessive connective tissue disorder characterized by skin fragility, delayed wound healing, joint hyperlaxity and contractures, muscle hypotonia, intellectual disability, and a spondyloepimetaphyseal dysplasia with bone fragility and severe kyphoscoliosis, identified biallelic B3GALT6 mutations, including homozygous missense mutations in family 1 (c.619G>C [p.Asp207His]) and family 3 (c.649G>A [p.Gly217Ser]) and compound heterozygous mutations in family 2 (c.323_344del [p.Ala108Glyfs(∗)163], c.619G>C [p.Asp207His]). The phenotype overlaps with several recessive Ehlers-Danlos variants and spondyloepimetaphyseal dysplasia with joint hyperlaxity. Affected individuals' fibroblasts exhibited a large decrease in ability to prime glycosaminoglycan synthesis together with impaired glycanation of the small chondroitin/dermatan sulfate proteoglycan decorin, confirming β3GalT6 loss of function. Dermal electron microcopy disclosed abnormalities in collagen fibril organization, in line with the important regulatory role of decorin in this process. A strong reduction in heparan sulfate level was also observed, indicating that β3GalT6 deficiency alters synthesis of both main types of glycosaminoglycans. In vitro wound healing assay revealed a significant delay in fibroblasts from two index individuals, pointing to a role for glycosaminoglycan defect in impaired wound repair in vivo. Our study emphasizes a crucial role for β3GalT6 in multiple major developmental and pathophysiological processes.
Copyright © 2013 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Mutations in B3GALT6, which encodes a glycosaminoglycan linker region enzyme, cause a spectrum of skeletal and connective tissue disorders.Am J Hum Genet. 2013 Jun 6;92(6):927-34. doi: 10.1016/j.ajhg.2013.04.003. Epub 2013 May 9. Am J Hum Genet. 2013. PMID: 23664117 Free PMC article.
-
Biallelic B3GALT6 mutations cause spondylodysplastic Ehlers-Danlos syndrome.Hum Mol Genet. 2018 Oct 15;27(20):3475-3487. doi: 10.1093/hmg/ddy234. Hum Mol Genet. 2018. PMID: 29931299
-
A B3GALT6 variant in patient originally described as Al-Gazali syndrome and implicating the endoplasmic reticulum quality control in the mechanism of some β3GalT6-pathy mutations.Clin Genet. 2018 Jun;93(6):1148-1158. doi: 10.1111/cge.13236. Epub 2018 Mar 15. Clin Genet. 2018. PMID: 29443383
-
Genetic heterogeneity and clinical variability in musculocontractural Ehlers-Danlos syndrome caused by impaired dermatan sulfate biosynthesis.Hum Mutat. 2015 May;36(5):535-47. doi: 10.1002/humu.22774. Epub 2015 Apr 6. Hum Mutat. 2015. PMID: 25703627 Review.
-
Ehlers-Danlos syndrome associated with glycosaminoglycan abnormalities.Adv Exp Med Biol. 2014;802:145-59. doi: 10.1007/978-94-007-7893-1_10. Adv Exp Med Biol. 2014. PMID: 24443026 Review.
Cited by
-
A phylogenetic view and functional annotation of the animal β1,3-glycosyltransferases of the GT31 CAZy family.Glycobiology. 2021 Apr 1;31(3):243-259. doi: 10.1093/glycob/cwaa086. Glycobiology. 2021. PMID: 32886776 Free PMC article.
-
Inherited Proteoglycan Biosynthesis Defects-Current Laboratory Tools and Bikunin as a Promising Blood Biomarker.Genes (Basel). 2021 Oct 20;12(11):1654. doi: 10.3390/genes12111654. Genes (Basel). 2021. PMID: 34828260 Free PMC article. Review.
-
Xylose phosphorylation functions as a molecular switch to regulate proteoglycan biosynthesis.Proc Natl Acad Sci U S A. 2014 Nov 4;111(44):15723-8. doi: 10.1073/pnas.1417993111. Epub 2014 Oct 20. Proc Natl Acad Sci U S A. 2014. PMID: 25331875 Free PMC article.
-
The Ehlers-Danlos syndromes.Nat Rev Dis Primers. 2020 Jul 30;6(1):64. doi: 10.1038/s41572-020-0194-9. Nat Rev Dis Primers. 2020. PMID: 32732924 Review.
-
Mutant B3GALT6 in a Multiplex Family: A Dominant Variant Co-Segregated With Moderate Malformations.Front Genet. 2022 Jun 6;13:824445. doi: 10.3389/fgene.2022.824445. eCollection 2022. Front Genet. 2022. PMID: 35734427 Free PMC article.
References
-
- Turnbull J.E. Heparan sulfate glycomics: towards systems biology strategies. Biochem. Soc. Trans. 2010;38:1356–1360. - PubMed
-
- Bishop J.R., Schuksz M., Esko J.D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. - PubMed
-
- Prydz K., Dalen K.T. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000;113:193–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

