Mass spectrometry techniques in the survey of steroid metabolites as potential disease biomarkers: a review
- PMID: 23664145
- PMCID: PMC3755027
- DOI: 10.1016/j.metabol.2013.04.003
Mass spectrometry techniques in the survey of steroid metabolites as potential disease biomarkers: a review
Abstract
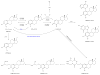
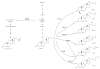
Mass spectrometric approaches have been fundamental to the identification of metabolites associated with steroid hormones, yet this topic has not been reviewed in depth in recent years. To this end, and given the increasing relevance of liquid chromatography-mass spectrometry (LC-MS) studies on steroid hormones and their metabolites, the present review addresses this subject. This review provides a timely summary of the use of various mass spectrometry-based analytical techniques during the evaluation of steroidal biomarkers in a range of human disease settings. The sensitivity and specificity of these technologies are clearly providing valuable new insights into breast cancer and cardiovascular disease. We aim to contribute to an enhanced understanding of steroid metabolism and how it can be profiled by LC-MS techniques.
Keywords: 1,2-Dihydroxybenzene (benzene catechol); 1,2-Dihydroxybenzene-Quinone; APCI–MS; APPI–MS; Atmospheric-pressure chemical ionization mass spectrometry; Atmospheric-pressure photoionization mass spectrometry; CA; CAT; CAT-Q; CID; COMT; Cancer biomarkers; Catechol-O-methyltransferase; Cholesterol metabolites; Cholic acid; Collision-induced dissociation; DCA; Deoxycholic acid; ESI–MS; Electrospray ionization; Estradiol metabolites; FIA; FTMS; Flow injection analysis; Fourier transform mass spectrometry; GC–MS; GP; Gas chromatography–mass spectrometry; Girard P; HPLC–EDC; High performance liquid chromatography–electro-chemical detection; LC–MS; Liquid chromatography–mass spectrometry; MALDI-TOF; Mass spectrometry; Matrix-assisted laser desorption/ionization-time-of-flight; N-AcCys; N-acetylcysteine; N-acetyldopamine; N-acetyldopamine-quinone; NADA; NADA-Q; NQO-2; NRH quinone oxidoreductase 2; Resv; Resveratrol; SLOS; SPE; SRM; Selected reaction monitoring; Smith–Lemli–Opitz syndrome; Solid phase extration; TOF; Time-of-flight; UPLC–MS/MS; Ultra-performance liquid chromatography-tandem mass spectrometry.
Crown Copyright © 2013. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
-
Critical practical aspects in the application of liquid chromatography-mass spectrometric studies for the characterization of impurities and degradation products.J Pharm Biomed Anal. 2014 Jan;87:191-217. doi: 10.1016/j.jpba.2013.04.027. Epub 2013 Apr 28. J Pharm Biomed Anal. 2014. PMID: 23706957 Review.
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
-
Androgen glucuronides analysis by liquid chromatography tandem-mass spectrometry: could it raise new perspectives in the diagnostic field of hormone-dependent malignancies?J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Dec 1;940:24-34. doi: 10.1016/j.jchromb.2013.09.022. Epub 2013 Sep 27. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 24140653 Review.
-
Comparative limitations and benefits of liquid chromatography - mass spectrometry techniques for analysis of sex steroids in tears.Exp Eye Res. 2019 Feb;179:168-178. doi: 10.1016/j.exer.2018.11.015. Epub 2018 Nov 15. Exp Eye Res. 2019. PMID: 30448340
Cited by
-
Carcinogenic liver fluke Opisthorchis viverrini oxysterols detected by LC-MS/MS survey of soluble fraction parasite extract.Parasitol Int. 2013 Dec;62(6):535-42. doi: 10.1016/j.parint.2013.08.001. Epub 2013 Aug 20. Parasitol Int. 2013. PMID: 23973383 Free PMC article.
-
High performance liquid chromatography: Tandem mass spectrometric determination of cisplatin levels in different visceral pleura layers of rats.Oncol Lett. 2015 May;9(5):2388-2392. doi: 10.3892/ol.2015.2989. Epub 2015 Feb 26. Oncol Lett. 2015. PMID: 26137076 Free PMC article.
-
Estradiol, Estrone and Ethinyl Estradiol Metabolism Studied by High Resolution LC-MS/MS Using Stable Isotope Labeling and Trapping of Reactive Metabolites.Metabolites. 2022 Sep 30;12(10):931. doi: 10.3390/metabo12100931. Metabolites. 2022. PMID: 36295833 Free PMC article.
-
Liquid chromatography-ion mobility spectrometry-mass spectrometry analysis of multiple classes of steroid hormone isomers in a mixture.J Chromatogr B Analyt Technol Biomed Life Sci. 2020 Jan 15;1137:121941. doi: 10.1016/j.jchromb.2019.121941. Epub 2019 Dec 16. J Chromatogr B Analyt Technol Biomed Life Sci. 2020. PMID: 31877426 Free PMC article.
-
Facilitating chemical and biochemical experiments with electronic microcontrollers and single-board computers.Nat Protoc. 2020 Mar;15(3):925-990. doi: 10.1038/s41596-019-0272-1. Epub 2020 Jan 29. Nat Protoc. 2020. PMID: 31996842
References
-
- Want EJ, Cravatt BF, Siuzdak G. The expanding role of mass spectrometry in metabolite profiling and characterization. ChemBioChem. 2005;6:1941–51. - PubMed
-
- Dear GJ, Plumb AR, Fraser IJ. The rapid identification of drug metabolites using capillary liquid chromatography coupled to an ion trap mass spectrometer. Rapid Commun Mass Spectrom. 1999;3:456–63. - PubMed
-
- Zhang N, Fountain ST, Bi H, Rossi ST. Quantification and rapid metabolite identification in drug discovery using API Time-of-Flight LC/MS. Anal Chem. 2000;72:800–6. - PubMed
-
- Shockor JP, Holmes E. Metabonomic Applications in Toxicity Screening and Disease Diagnosis. Current Trop Med Chem. 2002;2:35–51. - PubMed
-
- Plumb RS, Stumpf CL, Granger JH, Castro-Perez J, Haselden JN, Dear GJ. Use of liquid chromatography/time-of-flight mass spectrometry and multivariate statistical analysis shows promise for the detection of drug metabolites in biological fluids. Rapid Commun Mass Spectrom. 2003;17:2632–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

