Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors
- PMID: 23664153
- PMCID: PMC4177078
- DOI: 10.1016/j.ajo.2013.02.009
Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors
Abstract
Purpose: To report results of aflibercept therapy in eyes with neovascular age-related macular degeneration previously treated with bevacizumab, ranibizumab, or both.
Design: Retrospective, interventional, noncomparative, consecutive case series.
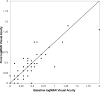
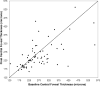
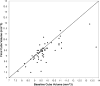
Methods: Ninety-six eyes from 85 patients with neovascular age-related macular degeneration who previously had received bevacizumab, ranibizumab, or both were treated with aflibercept monthly for 3 months followed by a fourth injection within 2 months. Outcomes were determined 4 ± 1 months after the first aflibercept dose and included: proportion of patients gaining or losing 2 lines or more of best-corrected visual acuity, proportion remaining within a gain or loss of 1 line, mean change in logarithm of the minimal angle of resolution visual acuity, mean change in central foveal thickness, mean change in macular cube volume, and qualitative anatomic response as assessed by spectral-domain optical coherence tomography.
Results: At baseline, 82 (85%) eyes had signs of active exudation despite a mean of 17 previous anti-vascular endothelial growth factor injections. At final visit, 82 (85%) remained stable within a gain or loss of 1 line, 7 (7%) gained 2 lines or more, and 7 (7%) lost 2 lines or more of best-corrected visual acuity. Mean logarithm of the minimal angle of resolution visual acuity showed minimal change 0.02 (range, -0.46 to 0.70; P = .14). Mean central foveal thickness decreased -18 μm (range, -242 to 198 μm; P = .06). Mean macular volume decreased -0.27 mm(3) (95% confidence interval, -0.4 to -0.1 mm(3); P = .004). On qualitative analysis, 4 (5%) eyes had complete resolution of exudative fluid, 40 (49%) showed partial resolution, 26 (32%) remained unchanged, and 12 (14%) showed worsened exudative fluid.
Conclusions: Aflibercept seems to be an effective alternative for neovascular age-related macular degeneration patients previously treated with bevacizumab, ranibizumab, or both at 4 months of follow-up. Most treated eyes demonstrated stable visual acuity and anatomic improvements by spectral-domain optical coherence tomography.
Published by Elsevier Inc.
Figures
References
-
- Friedman DS, O’Colmain BJ, Munoz B, et al. The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. - PubMed
-
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291(15):1900–1901. - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. - PubMed
-
- Rosenfeld PG, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. - PubMed
-
- Avery RL, Pieramici DJ, Rabena MD, Casterllarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(3):363–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

