Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials
- PMID: 23664333
- PMCID: PMC6083850
- DOI: 10.1016/S1473-3099(13)70093-8
Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials
Abstract
Background: Two randomised, placebo-controlled trials-BENCHMRK-1 and BENCHMRK-2-investigated the efficacy and safety of raltegravir, an HIV-1 integrase strand-transfer inhibitor. We report final results of BENCHMRK-1 and BENCHMRK-2 combined at 3 years (the end of the double-blind phase) and 5 years (the end of the study).
Methods: Integrase-inhibitor-naive patients with HIV resistant to three classes of drug and who were failing antiretroviral therapy were enrolled. Patients were randomly assigned (2:1) to raltegravir 400 mg twice daily or placebo, both with optimised background treatment. Patients and investigators were masked to treatment allocation until week 156, after which all patients were offered open-label raltegravir until week 240. The primary endpoint was previously assessed at 16 weeks. We assessed long-term efficacy with endpoints of the proportion of patients with an HIV viral load of less than 50 copies per mL and less than 400 copies per mL, and mean change in CD4 cell count, at weeks 156 and 240.
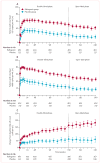
Findings: 1012 patients were screened for inclusion. 462 were treated with raltegravir and 237 with placebo. At week 156, 51% in the raltegravir group versus 22% in the placebo group (non-completer classed as failure) had viral loads of less than 50 copies per mL, and 54% versus 23% had viral loads of less than 400 copies per mL. Mean CD4 cell count increase (analysed by an observed failure approach) was 164 cells per μL versus 63 cells per μL. After week 156, 251 patients (54%) from the raltegravir group and 47 (20%) from the placebo group entered the open-label raltergravir phase; 221 (47%) versus 44 (19%) completed the entire study. At week 240, viral load was less than 50 copies per mL in 193 (42%) of all patients initially assigned to raltegravir and less than 400 copies per mL in 210 (45%); mean CD4 cell count increased by 183 cells per μL. Virological failure occurred in 166 raltegravir recipients (36%) during the double-blind phase and in 17 of all patients (6%) during the open-label phase. The most common drug-related adverse events at 5 years in both groups were nausea, headache, and diarrhoea, and occurred in similar proportions in each group. Laboratory test results were similar in both treatment groups and showed little change after year 2.
Interpretation: Raltegravir has a favourable long-term efficacy and safety profile in integrase-inhibitor-naive patients with triple-class resistant HIV in whom antiretroviral therapy is failing. Raltegravir is an alternative for treatment-experienced patients, particularly those with few treatment options.
Funding: Merck Sharp & Dohme.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
JJE has received research support from Merck, GlaxoSmithKline/ViiV Healthcare, and Bristol-Myers Squibb, and consulting fees from Merck, Bristol-Myers Squibb, GlaxoSmithKline/ViiV Healthcare, Gilead Sciences, Janssen Pharmaceuticals. DAC has received research support, speaker fees, and consulting fees from Merck. RTS has received research support, speaker fees, and consulting fees from Merck. BC has been a consultant on advisory boards, participated in speakers’ bureaus, or done clinical trials with Roche, Boehringer-Ingelheim, Abbott, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, Tibotec, Janssen Pharmaceuticals, Merck, Pfizer, Siemens, Monogram Biosciences, and Panacos. JMG has received research support, speaker fees, and consulting fees from Merck. PNK has been an investigator for Merck, GlaxoSmithKline, Janssen Pharmaceuticals, and Bristol-Myers Squibb; a paid consultant for Bristol-Myers Squibb, ViiV Healthcare, and Janssen; has received speaker fees from Janssen Pharmaceuticals and ViiV Healthcare; and owns stock in Pfizer, GlaxoSmithKline, Gilead Sciences, and Janssen Pharmaceuticals. JKR has received honoraria for lectures or participation in advisory boards from Merck, Roche, GlaxoSmithKline, Bristol-Myers Squibb, Tibotec, Pfizer, Gilead Sciences, Abbott, ViiV Healthcare, and Boehringer Ingelheim. MS has received research support from Merck, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences; and has acted as a speaker and consulted for Abbott, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, and Merck. MM has received research support from Merck, Gilead Sciences, GlaxoSmithKline, and Tobira; speaker fees from Gilead Sciences and Tibotec; and consulting fees from Merck, Gilead Sciences, Tibotec, and ViiV Healthcare. MRL has received research support from Merck. AL has been an adviser for Merck Sharp & Dohme, GlaxoSmithKline, Bristol-Myers Squibb, Gilead Sciences, Monogram, Abbott, and Tibotec; has received lecture fees from Merck Sharp & Dohme, Bristol-Myers Squibb, Abbott, Pfizer, Roche, and Boehringer-Ingelheim; and has received research support from Merck Sharp & Dohme, GlaxoSmithKline, Bristol-Myers Squibb, Gilead Sciences, Pfizer, Roche, and Schering-Plough. JLL has received research support and speaker’s fees from Merck, and has served on Merck’s antiretroviral scientific advisory board. He has also received research support from Gilead, GlaxoSmith Kline, and Pfizer. KMS, HW, RJOB, BTN, and HT are current or former employees of Merck Sharp & Dohme, a subsidiary of Merck, and own stock or stock options in the company. PY declares that he has no conflicts of interest.
Figures
Comment in
-
A benchmark for management of drug resistant HIV.Lancet Infect Dis. 2013 Jul;13(7):561-562. doi: 10.1016/S1473-3099(13)70113-0. Epub 2013 May 7. Lancet Infect Dis. 2013. PMID: 23664332 No abstract available.
References
-
- Hazuda DJ, Felock P, Witmer M, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000;287:646–50. - PubMed
-
- Merck Prescribing information for ISENTRESS (raltegravir) tablets
-
- Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–54. - PubMed
-
- Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359:355–65. - PubMed
-
- Steigbigel RT, Cooper DA, Teppler H, et al. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 phase III trials. Clin Infect Dis. 2010;50:605–12. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

