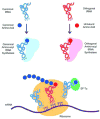
Protein conjugation with genetically encoded unnatural amino acids
- PMID: 23664497
- PMCID: PMC4284959
- DOI: 10.1016/j.cbpa.2013.04.017
Protein conjugation with genetically encoded unnatural amino acids
Abstract
The site-specific incorporation of unnatural amino acids with orthogonal chemical reactivity into proteins enables the synthesis of structurally defined protein conjugates. Amino acids containing ketone, azide, alkyne, alkene, and tetrazine side chains can be genetically encoded in response to nonsense and frameshift codons. These bio-orthogonal chemical handles allow precise control over the site and stoichiometry of conjugation, and have enabled medicinal chemistry-like optimization of the physical and biological properties of protein conjugates, especially the next-generation protein therapeutics.
Copyright © 2013. Published by Elsevier Ltd.
Figures
Similar articles
-
Therapeutic applications of an expanded genetic code.Chembiochem. 2014 Aug 18;15(12):1721-9. doi: 10.1002/cbic.201402154. Epub 2014 Jul 18. Chembiochem. 2014. PMID: 25044800 Free PMC article. Review.
-
Antibody conjugates with unnatural amino acids.Mol Pharm. 2015 Jun 1;12(6):1848-62. doi: 10.1021/acs.molpharmaceut.5b00082. Epub 2015 May 21. Mol Pharm. 2015. PMID: 25898256 Review.
-
Synthesis of site-specific antibody-drug conjugates using unnatural amino acids.Proc Natl Acad Sci U S A. 2012 Oct 2;109(40):16101-6. doi: 10.1073/pnas.1211023109. Epub 2012 Sep 17. Proc Natl Acad Sci U S A. 2012. PMID: 22988081 Free PMC article.
-
Selective Protein Degradation through Tetrazine Ligation of Genetically Incorporated Unnatural Amino Acids.Chem Asian J. 2024 Dec 2;19(23):e202400824. doi: 10.1002/asia.202400824. Epub 2024 Oct 26. Chem Asian J. 2024. PMID: 39221720
-
Direct protein-protein conjugation by genetically introducing bioorthogonal functional groups into proteins.Bioorg Med Chem. 2016 Nov 15;24(22):5816-5822. doi: 10.1016/j.bmc.2016.09.035. Epub 2016 Sep 15. Bioorg Med Chem. 2016. PMID: 27670101
Cited by
-
Conformation-specific detection of calmodulin binding using the unnatural amino acid p-azido-phenylalanine (AzF) as an IR-sensor.Struct Dyn. 2018 Nov 7;5(6):064701. doi: 10.1063/1.5053466. eCollection 2018 Nov. Struct Dyn. 2018. PMID: 30474048 Free PMC article.
-
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast.ACS Synth Biol. 2018 Sep 21;7(9):2256-2269. doi: 10.1021/acssynbio.8b00260. Epub 2018 Sep 4. ACS Synth Biol. 2018. PMID: 30139255 Free PMC article.
-
One-Pot Dual Labeling of IgG 1 and Preparation of C-to-C Fusion Proteins Through a Combination of Sortase A and Butelase 1.Bioconjug Chem. 2018 Oct 17;29(10):3245-3249. doi: 10.1021/acs.bioconjchem.8b00563. Epub 2018 Sep 21. Bioconjug Chem. 2018. PMID: 30231608 Free PMC article.
-
Protein PEGylation for cancer therapy: bench to bedside.J Cell Commun Signal. 2019 Sep;13(3):319-330. doi: 10.1007/s12079-018-0492-0. Epub 2018 Nov 29. J Cell Commun Signal. 2019. PMID: 30499020 Free PMC article. Review.
-
Methods for site-specific drug conjugation to antibodies.MAbs. 2014 Jan-Feb;6(1):46-53. doi: 10.4161/mabs.26632. MAbs. 2014. PMID: 24135651 Free PMC article. Review.
References
-
- Basle E, Joubert N, Pucheault M. Protein chemical modification on endogenous amino acids. Chemistry & biology. 2010;17(3):213–227. - PubMed
-
- O’Hare HM, Johnsson K, Gautier A. Chemical probes shed light on protein function. Current opinion in structural biology. 2007;17(4):488–494. - PubMed
-
- Muir TW. Semisynthesis of proteins by expressed protein ligation. Annual review of biochemistry. 2003;72:249–289. - PubMed
-
- Liu CC, Schultz PG. Adding new chemistries to the genetic code. Annual review of biochemistry. 2010;79:413–444. This comprehensive review describes genetic incorporation and function of a wide range of unnatural amino acids. - PubMed
-
- Hendrickson TL, de Crecy-Lagard V, Schimmel P. Incorporation of nonnatural amino acids into proteins. Annual review of biochemistry. 2004;73:147–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources