Temporal processing in the olfactory system: can we see a smell?
- PMID: 23664611
- PMCID: PMC3694266
- DOI: 10.1016/j.neuron.2013.04.033
Temporal processing in the olfactory system: can we see a smell?
Abstract
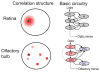
Sensory processing circuits in the visual and olfactory systems receive input from complex, rapidly changing environments. Although patterns of light and plumes of odor create different distributions of activity in the retina and olfactory bulb, both structures use what appears on the surface similar temporal coding strategies to convey information to higher areas in the brain. We compare temporal coding in the early stages of the olfactory and visual systems, highlighting recent progress in understanding the role of time in olfactory coding during active sensing by behaving animals. We also examine studies that address the divergent circuit mechanisms that generate temporal codes in the two systems, and find that they provide physiological information directly related to functional questions raised by neuroanatomical studies of Ramon y Cajal over a century ago. Consideration of differences in neural activity in sensory systems contributes to generating new approaches to understand signal processing.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Understanding smell--the olfactory stimulus problem.Neurosci Biobehav Rev. 2013 Sep;37(8):1667-79. doi: 10.1016/j.neubiorev.2013.06.009. Epub 2013 Jun 25. Neurosci Biobehav Rev. 2013. PMID: 23806440 Review.
-
Second-order neurones and receptor mechanisms in visual- and olfactory-information processing.Trends Neurosci. 1995 Aug;18(8):359-64. doi: 10.1016/0166-2236(95)93929-r. Trends Neurosci. 1995. PMID: 7482799 Review.
-
Coding and transformations in the olfactory system.Annu Rev Neurosci. 2014;37:363-85. doi: 10.1146/annurev-neuro-071013-013941. Epub 2014 Jun 2. Annu Rev Neurosci. 2014. PMID: 24905594 Review.
-
Neural correlates of olfactory learning: Critical role of centrifugal neuromodulation.Learn Mem. 2010 Oct 27;17(11):561-70. doi: 10.1101/lm.941510. Print 2010 Nov. Learn Mem. 2010. PMID: 20980444 Free PMC article.
-
Inhalation Frequency Controls Reformatting of Mitral/Tufted Cell Odor Representations in the Olfactory Bulb.J Neurosci. 2018 Feb 28;38(9):2189-2206. doi: 10.1523/JNEUROSCI.0714-17.2018. Epub 2018 Jan 26. J Neurosci. 2018. PMID: 29374137 Free PMC article.
Cited by
-
Physical principles for scalable neural recording.Front Comput Neurosci. 2013 Oct 21;7:137. doi: 10.3389/fncom.2013.00137. eCollection 2013. Front Comput Neurosci. 2013. PMID: 24187539 Free PMC article.
-
Assessment of direct knowledge of the human olfactory system.Exp Neurol. 2020 Jul;329:113304. doi: 10.1016/j.expneurol.2020.113304. Epub 2020 Apr 9. Exp Neurol. 2020. PMID: 32278646 Free PMC article. Review. No abstract available.
-
Plasticity of Sniffing Pattern and Neural Activity in the Olfactory Bulb of Behaving Mice During Odor Sampling, Anticipation, and Reward.Neurosci Bull. 2020 Jun;36(6):598-610. doi: 10.1007/s12264-019-00463-9. Epub 2020 Jan 27. Neurosci Bull. 2020. PMID: 31989425 Free PMC article.
-
Dense distributed processing in a hindlimb scratch motor network.J Neurosci. 2014 Aug 6;34(32):10756-64. doi: 10.1523/JNEUROSCI.1079-14.2014. J Neurosci. 2014. PMID: 25100606 Free PMC article.
-
An assistive computer vision tool to automatically detect changes in fish behavior in response to ambient odor.Sci Rep. 2021 Jan 13;11(1):1002. doi: 10.1038/s41598-020-79772-3. Sci Rep. 2021. PMID: 33441714 Free PMC article.
References
-
- Abbott LF, Rolls ET, Tovee MJ. Representational capacity of face coding in monkeys. Cereb Cortex. 1996;6:498–505. - PubMed
-
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. - PubMed
-
- Alonso M, Lepousez G, Sebastien W, Bardy C, Gabellec MM, Torquet N, Lledo PM. Activation of adult-born neurons facilitates learning and memory. Nat Neurosci. 2012;15:897–904. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

