Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis
- PMID: 23664616
- PMCID: PMC3690324
- DOI: 10.1016/j.neuron.2013.03.017
Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis
Abstract
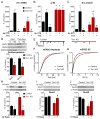
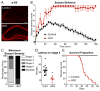
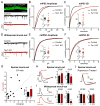
Neural circuits are regulated by activity-dependent feedback systems that tightly control network excitability and which are thought to be crucial for proper brain development. Defects in the ability to establish and maintain network homeostasis may be central to the pathogenesis of neurodevelopmental disorders. Here, we examine the function of the tuberous sclerosis complex (TSC)-mTOR signaling pathway, a common target of mutations associated with epilepsy and autism spectrum disorder, in regulating activity-dependent processes in the mouse hippocampus. We find that the TSC-mTOR pathway is a central component of a positive feedback loop that promotes network activity by repressing inhibitory synapses onto excitatory neurons. In Tsc1 KO neurons, weakened inhibition caused by deregulated mTOR alters the balance of excitatory and inhibitory synaptic transmission, leading to hippocampal hyperexcitability. These findings identify the TSC-mTOR pathway as a regulator of neural network activity and have implications for the neurological dysfunction in disorders exhibiting deregulated mTOR signaling.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


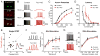

References
-
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. - PubMed
-
- Chevere-Torres I, Kaphzan H, Bhattacharya A, Kang A, Maki JM, Gambello MJ, Arbiser JL, Santini E, Klann E. Metabotropic glutamate receptor-dependent long-term depression is impaired due to elevated ERK signaling in the DeltaRG mouse model of tuberous sclerosis complex. Neurobiol Dis. 2012;45:1101–1110. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

