Disorders of protein misfolding: alpha-1-antitrypsin deficiency as prototype
- PMID: 23664631
- PMCID: PMC3725216
- DOI: 10.1016/j.jpeds.2013.03.077
Disorders of protein misfolding: alpha-1-antitrypsin deficiency as prototype
Keywords: AT; ATD; ATM; ATZ; Alpha-1-antitrypsin; Alpha-1-antitrypsin deficiency; CBZ; COPD; Carbamazepine; Chronic obstructive pulmonary disease; ER; Endoplasmic reticulum; FDA; Federal Drug Administration; Mutant alpha-1-antitrypsin Z; Normal (wild type) alpha-1-antitrypsin; PBA; Phenylbutyric acid; RNA interference; RNAi; SNP; Single nucleotide polymorphism.
Conflict of interest statement
The other authors declare no conflicts of interest.
Figures

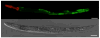
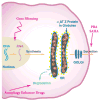
References
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Perlmutter DH. Alpha-1-antitrypsin deficiency: importance of proteasomal and autophagic degradative pathways in disposal of liver disease-associated protein aggregates. Annu Rev Med. 2011;62:333–345. - PubMed
-
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med. 2009;360:2749–2757. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

