Locomotion controls spatial integration in mouse visual cortex
- PMID: 23664971
- PMCID: PMC3661981
- DOI: 10.1016/j.cub.2013.04.012
Locomotion controls spatial integration in mouse visual cortex
Abstract
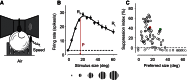
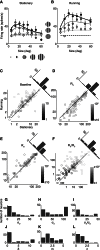
Growing evidence indicates that responses in sensory cortex are modulated by factors beyond direct sensory stimulation. In primary visual cortex (V1), for instance, responses increase with locomotion. Here we show that this increase is accompanied by a profound change in spatial integration. We recorded from V1 neurons in head-fixed mice placed on a spherical treadmill. We characterized spatial integration and found that the responses of most neurons were suppressed by large stimuli. As in primates, this surround suppression increased with stimulus contrast. These effects were captured by a divisive normalization model, where the numerator originates from a central region driving the neuron and the denominator originates from a larger suppressive field. We then studied the effects of locomotion and found that it markedly reduced surround suppression, allowing V1 neurons to integrate over larger regions of visual space. Locomotion had two main effects: it increased spontaneous activity, and it weakened the suppressive signals mediating normalization, relative to the driving signals. We conclude that a fundamental aspect of visual processing, spatial integration, is controlled by an apparently unrelated factor, locomotion. This control might operate through the mechanisms that are in place to deliver surround suppression.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
-
- Ghazanfar A.A., Schroeder C.E. Is neocortex essentially multisensory? Trends Cogn. Sci. 2006;10:278–285. - PubMed
-
- Matyas F., Sreenivasan V., Marbach F., Wacongne C., Barsy B., Mateo C., Aronoff R., Petersen C.C. Motor control by sensory cortex. Science. 2010;330:1240–1243. - PubMed
-
- Shuler M.G., Bear M.F. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

