Identification and distribution of projections from monoaminergic and cholinergic nuclei to functionally differentiated subregions of prefrontal cortex
- PMID: 23665053
- PMCID: PMC3811940
- DOI: 10.1016/j.brainres.2013.04.057
Identification and distribution of projections from monoaminergic and cholinergic nuclei to functionally differentiated subregions of prefrontal cortex
Abstract
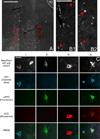
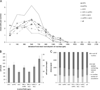
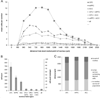
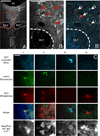
The prefrontal cortex (PFC) is implicated in a variety of cognitive and executive functions and is composed of several distinct networks, including anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC), and orbitofrontal cortex (OFC). These regions serve dissociable cognitive functions, and are heavily innervated by acetylcholine, dopamine, serotonin and norepinephrine systems. In this study, fluorescently labeled retrograde tracers were injected into the ACC, mPFC, and OFC, and labeled cells were identified in the nucleus basalis (NB), ventral tegmental area (VTA), dorsal raphe nucleus (DRN) and locus coeruleus (LC). DRN and LC showed similar distributions of retrogradely labeled neurons such that most were single labeled and the largest population projected to mPFC. VTA showed a slightly greater proportion of double and triple labeled neurons, with the largest population projecting to OFC. NB, on the other hand, showed mostly double and triple labeled neurons projecting to multiple subregions. Therefore, subsets of VTA, DRN and LC neurons may be capable of modulating individual prefrontal subregions independently, whereas NB cells may exert a more unified influence on the three areas simultaneously. These findings emphasize the unique aspects of the cholinergic and monoaminergic projections to functionally and anatomically distinct subregions of PFC.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures





References
-
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann. N. Y. Acad. Sci. 2004;1018:46–57. - PubMed
-
- Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J. Comp. Neurol. 1997;378(4):454–167. - PubMed
-
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 2005a;493(1):99–110. - PubMed
-
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005b;28:403–450. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

