Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome
- PMID: 23665154
- PMCID: PMC3775123
- DOI: 10.1016/j.biocel.2013.04.023
Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome
Abstract
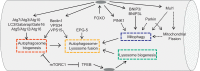
Skeletal muscle adapts its mass as consequence of physical activity, metabolism and hormones. Catabolic conditions or inactivity induce signaling pathways that regulate the process of muscle loss. Muscle atrophy in adult tissue occurs when protein degradation rates exceed protein synthesis. Two major protein degradation pathways, the ubiquitin-proteasome and the autophagy-lysosome systems, are activated during muscle atrophy and variably contribute to the loss of muscle mass. These degradation systems are controlled by a transcription dependent program that modulates the expression of rate-limiting enzymes of these proteolytic systems. The transcription factors FoxO, which are negatively regulated by Insulin-Akt pathway, and NF-κB, which is activated by inflammatory cytokines, were the first to be identified as critical for the atrophy process. In the last years a variety of pathways and transcription factors have been found to be involved in regulation of atrophy. This review will focus on the last progress in ubiquitin-proteasome and autophagy-lysosome systems and their involvement in muscle atrophy. This article is part of a Directed Issue entitled: Molecular basis of muscle wasting.
Keywords: Atrophy; Autophagy; FoxO; Muscle wasting; Skeletal muscle; Ubiquitin protesaome.
Copyright © 2013 The Author. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Arndt V., Dick N., Tawo R., Dreiseidler M., Wenzel D., Hesse M. Chaperone-assisted selective autophagy is essential for muscle maintenance. Current Biology. 2010;20:143–148. - PubMed
-
- Bdolah Y., Segal A., Tanksale P., Karumanchi S.A., Lecker S.H. Atrophy-related ubiquitin ligases atrogin-1 and MuRF-1 are associated with uterine smooth muscle involution in the postpartum period. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2007;292:R971–R976. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

