The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction
- PMID: 23665224
- PMCID: PMC3671584
- DOI: 10.1016/j.celrep.2013.04.013
The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction
Abstract
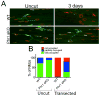
Axon degeneration is an evolutionarily conserved process that drives the loss of damaged axons and is an early event in many neurological disorders, so it is important to identify the molecular constituents of this poorly understood mechanism. Here, we demonstrate that the Phr1 E3 ubiquitin ligase is a central component of this axon degeneration program. Loss of Phr1 results in prolonged survival of severed axons in both the peripheral and central nervous systems, as well as preservation of motor and sensory nerve terminals. Phr1 depletion increases the axonal level of the axon survival molecule nicotinamide mononucleotide adenyltransferase 2 (NMNAT2), and NMNAT2 is necessary for Phr1-dependent axon stability. The profound long-term protection of peripheral and central mammalian axons following Phr1 deletion suggests that pharmacological inhibition of Phr1 function may be an attractive therapeutic candidate for amelioration of axon loss in neurological disease.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Beirowski B, Berek L, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. Quantitative and qualitative analysis of Wallerian degeneration using restricted axonal labelling in YFP-H mice. J Neurosci Methods. 2004;134:23–35. - PubMed
-
- Beirowski B, Gustin J, Armour SM, Yamamoto H, Viader A, North BJ, Michan S, Baloh RH, Golden JP, Schmidt RE, et al. Sir-two-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling. Proc Natl Acad Sci U S A. 2012;108:E952–961. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

