Mechanism-based corrector combination restores ΔF508-CFTR folding and function
- PMID: 23666117
- PMCID: PMC3840170
- DOI: 10.1038/nchembio.1253
Mechanism-based corrector combination restores ΔF508-CFTR folding and function
Abstract
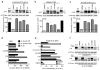
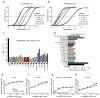
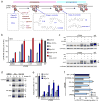
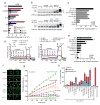
The most common cystic fibrosis mutation, ΔF508 in nucleotide binding domain 1 (NBD1), impairs cystic fibrosis transmembrane conductance regulator (CFTR)-coupled domain folding, plasma membrane expression, function and stability. VX-809, a promising investigational corrector of ΔF508-CFTR misprocessing, has limited clinical benefit and an incompletely understood mechanism, hampering drug development. Given the effect of second-site suppressor mutations, robust ΔF508-CFTR correction most likely requires stabilization of NBD1 energetics and the interface between membrane-spanning domains (MSDs) and NBD1, which are both established primary conformational defects. Here we elucidate the molecular targets of available correctors: class I stabilizes the NBD1-MSD1 and NBD1-MSD2 interfaces, and class II targets NBD2. Only chemical chaperones, surrogates of class III correctors, stabilize human ΔF508-NBD1. Although VX-809 can correct missense mutations primarily destabilizing the NBD1-MSD1/2 interface, functional plasma membrane expression of ΔF508-CFTR also requires compounds that counteract the NBD1 and NBD2 stability defects in cystic fibrosis bronchial epithelial cells and intestinal organoids. Thus, the combination of structure-guided correctors represents an effective approach for cystic fibrosis therapy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

