Immune-suppressive properties of the tumor microenvironment
- PMID: 23666510
- PMCID: PMC11029603
- DOI: 10.1007/s00262-013-1434-6
Immune-suppressive properties of the tumor microenvironment
Abstract
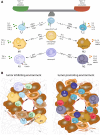
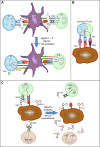
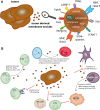
Solid tumors are more than an accumulation of cancer cells. Indeed, cancerous cells create a permissive microenvironment by exploiting non-transformed host cells. Thus, solid tumors rather resemble abnormal organs composed of the cancerous cells itself and the stroma providing the supportive framework. The stroma can be divided into the extracellular matrix consisting of proteoglycans, hyaluronic acid, and fibrous proteins, as well as stromal cells including mesenchymal and immune cells; moreover, it contains various peptide factors and metabolites. Here, we will focus on immune-modulating capacities of the tumor microenvironment.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

