Ribosomally synthesized and post-translationally modified peptide natural products: new insights into the role of leader and core peptides during biosynthesis
- PMID: 23666908
- PMCID: PMC3838977
- DOI: 10.1002/chem.201300401
Ribosomally synthesized and post-translationally modified peptide natural products: new insights into the role of leader and core peptides during biosynthesis
Abstract
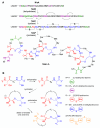
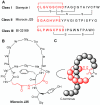
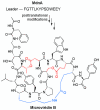
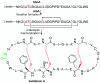
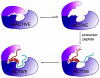
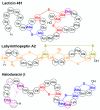
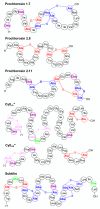
Ribosomally synthesized and post-translationally modified peptides (RiPPs) are a major class of natural products with a high degree of structural diversity and a wide variety of bioactivities. Understanding the biosynthetic machinery of these RiPPs will benefit the discovery and development of new molecules with potential pharmaceutical applications. In this Concept article, we discuss the features of the biosynthetic pathways to different RiPP classes, and propose mechanisms regarding recognition of the precursor peptide by the post-translational modification enzymes. We propose that the leader peptides function as allosteric regulators that bind the active form of the biosynthetic enzymes in a conformational selection process. We also speculate how enzymes that generate polycyclic products of defined topologies may have been selected for during evolution.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





References
-
- Arnison PGB, M. J., Bierbaum G, Bowers AA, Bulaj G, Camarero JA, Campopiano DJ, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian K-D, Fischbach MA, Garavelli JS, Göransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Reaney MJT, Rebuffat S, Ross RP, Sahl H-G, Saris P, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Süssmuth RE, Tagg JR, Tang G-L, Vederas JC, Walsh CT, Walton JD, Willey JM, van der Donk WA. Nat. Prod. Rep. 2013;30:108–160. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

