Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning
- PMID: 23667055
- PMCID: PMC3695366
- DOI: 10.1182/blood-2012-10-463414
Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning
Abstract
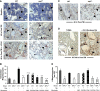
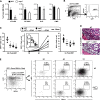
Successful hematopoietic stem cell (HSC) transplantation requires donor HSC engraftment within specialized bone marrow microenvironments known as HSC niches. We have previously reported a profound remodeling of the endosteal osteoblastic HSC niche after total body irradiation (TBI), defined as relocalization of surviving megakaryocytes to the niche site and marked expansion of endosteal osteoblasts. We now demonstrate that host megakaryocytes function critically in expansion of the endosteal niche after preparative radioablation and in the engraftment of donor HSC. We show that TBI-induced migration of megakaryocytes to the endosteal niche depends on thrombopoietin signaling through the c-MPL receptor on megakaryocytes, as well as CD41 integrin-mediated adhesion. Moreover, niche osteoblast proliferation post-TBI required megakaryocyte-secreted platelet-derived growth factor-BB. Furthermore, blockade of c-MPL-dependent megakaryocyte migration and function after TBI resulted in a significant decrease in donor HSC engraftment in primary and competitive secondary transplantation assays. Finally, we administered thrombopoietin to mice beginning 5 days before marrow radioablation and ending 24 hours before transplant to enhance megakaryocyte function post-TBI, and found that this strategy significantly enhanced donor HSC engraftment, providing a rationale for improving hematopoietic recovery and perhaps overall outcome after clinical HSC transplantation.
Figures




References
-
- Escalón MP, Komanduri KV. Cord blood transplantation: evolving strategies to improve engraftment and immune reconstitution. Curr Opin Oncol. 2010;22(2):122–129. - PubMed
-
- de la Morena MT, Gatti RA. A history of bone marrow transplantation. Immunol Allergy Clin North Am. 2010;30(1):1–15. - PubMed
-
- Garrett RW, Emerson SG. Bone and blood vessels: the hard and the soft of hematopoietic stem cell niches. Cell Stem Cell. 2009;4(6):503–506. - PubMed
-
- Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. - PubMed
-
- Xie Y, Yin T, Wiegraebe W, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457(7225):97–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

