HipA-triggered growth arrest and β-lactam tolerance in Escherichia coli are mediated by RelA-dependent ppGpp synthesis
- PMID: 23667235
- PMCID: PMC3697643
- DOI: 10.1128/JB.02210-12
HipA-triggered growth arrest and β-lactam tolerance in Escherichia coli are mediated by RelA-dependent ppGpp synthesis
Abstract
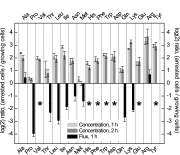
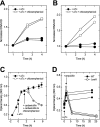
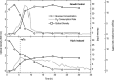
Persistence is a phenomenon whereby a subpopulation of bacterial cells enters a transient growth-arrested state that confers antibiotic tolerance. While entrance into persistence has been linked to the activities of toxin proteins, the molecular mechanisms by which toxins induce growth arrest and the persistent state remain unclear. Here, we show that overexpression of the protein kinase HipA in Escherichia coli triggers growth arrest by activating synthesis of the alarmone guanosine tetraphosphate (ppGpp) by the enzyme RelA, a signal typically associated with amino acid starvation. We further demonstrate that chemically suppressing ppGpp synthesis with chloramphenicol relieves inhibition of DNA replication initiation and RNA synthesis in HipA-arrested cells and restores vulnerability to β-lactam antibiotics. HipA-arrested cells maintain glucose uptake and oxygen consumption and accumulate amino acids as a consequence of translational inhibition. We harness the active metabolism of HipA-arrested cells to provide a bacteriophage-resistant platform for the production of biotechnologically relevant compounds, which may represent an innovative solution to the costly problem of phage contamination in industrial fermentations.
Figures


References
-
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622–1625 - PubMed
-
- Lewis K. 2010. Persister cells. Annu. Rev. Microbiol. 64:357–372 - PubMed
-
- Gerdes K, Maisonneuve E. 2012. Bacterial persistence and toxin-antitoxin loci. Annu. Rev. Microbiol. 66:103–123 - PubMed
-
- Yamaguchi Y, Inouye M. 2011. Regulation of growth and death in Escherichia coli by toxin-antitoxin systems. Nat. Rev. Microbiol. 9:779–790 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

