A highly productive, whole-cell DERA chemoenzymatic process for production of key lactonized side-chain intermediates in statin synthesis
- PMID: 23667462
- PMCID: PMC3647077
- DOI: 10.1371/journal.pone.0062250
A highly productive, whole-cell DERA chemoenzymatic process for production of key lactonized side-chain intermediates in statin synthesis
Abstract
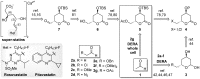
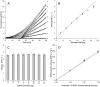
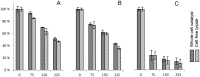
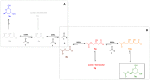
Employing DERA (2-deoxyribose-5-phosphate aldolase), we developed the first whole-cell biotransformation process for production of chiral lactol intermediates useful for synthesis of optically pure super-statins such as rosuvastatin and pitavastatin. Herein, we report the development of a fed-batch, high-density fermentation with Escherichia coli BL21 (DE3) overexpressing the native E. coli deoC gene. High activity of this biomass allows direct utilization of the fermentation broth as a whole-cell DERA biocatalyst. We further show a highly productive bioconversion processes with this biocatalyst for conversion of 2-substituted acetaldehydes to the corresponding lactols. The process is evaluated in detail for conversion of acetyloxy-acetaldehyde with the first insight into the dynamics of reaction intermediates, side products and enzyme activity, allowing optimization of the feeding strategy of the aldehyde substrates for improved productivities, yields and purities. The resulting process for production of ((2S,4R)-4,6-dihydroxytetrahydro-2H-pyran-2-yl)methyl acetate (acetyloxymethylene-lactol) has a volumetric productivity exceeding 40 g L(-1) h(-1) (up to 50 g L(-1) h(-1)) with >80% yield and >80% chromatographic purity with titers reaching 100 g L(-1). Stereochemical selectivity of DERA allows excellent enantiomeric purities (ee >99.9%), which were demonstrated on downstream advanced intermediates. The presented process is highly cost effective and environmentally friendly. To our knowledge, this is the first asymmetric aldol condensation process achieved with whole-cell DERA catalysis and it simplifies and extends previously developed DERA-catalyzed approaches based on the isolated enzyme. Finally, applicability of the presented process is demonstrated by efficient preparation of a key lactol precursor, which fits directly into the lactone pathway to optically pure super-statins.
Conflict of interest statement
Figures



Similar articles
-
Current state of and need for enzyme engineering of 2-deoxy-D-ribose 5-phosphate aldolases and its impact.Appl Microbiol Biotechnol. 2021 Aug;105(16-17):6215-6228. doi: 10.1007/s00253-021-11462-0. Epub 2021 Aug 19. Appl Microbiol Biotechnol. 2021. PMID: 34410440 Free PMC article. Review.
-
Engineered, highly productive biosynthesis of artificial, lactonized statin side-chain building blocks: The hidden potential of Escherichia coli unleashed.Metab Eng. 2014 Jul;24:160-72. doi: 10.1016/j.ymben.2014.05.012. Epub 2014 May 21. Metab Eng. 2014. PMID: 24858788
-
The fed-batch production of a thermophilic 2-deoxyribose-5-phosphate aldolase (DERA) in Escherichia coli by exponential feeding strategy control.Appl Biochem Biotechnol. 2010 Nov;162(5):1423-34. doi: 10.1007/s12010-010-8924-1. Epub 2010 Mar 15. Appl Biochem Biotechnol. 2010. PMID: 20229283
-
Characterization and application of a newly synthesized 2-deoxyribose-5-phosphate aldolase.J Ind Microbiol Biotechnol. 2013 Jan;40(1):29-39. doi: 10.1007/s10295-012-1213-y. Epub 2012 Nov 22. J Ind Microbiol Biotechnol. 2013. PMID: 23179467
-
2-Deoxy-D-ribose-5-phosphate aldolase (DERA): applications and modifications.Appl Microbiol Biotechnol. 2018 Dec;102(23):9959-9971. doi: 10.1007/s00253-018-9392-8. Epub 2018 Oct 3. Appl Microbiol Biotechnol. 2018. PMID: 30284013 Free PMC article. Review.
Cited by
-
Enhancing the biocatalytic manufacture of the key intermediate of atorvastatin by focused directed evolution of halohydrin dehalogenase.Sci Rep. 2017 Feb 6;7:42064. doi: 10.1038/srep42064. Sci Rep. 2017. PMID: 28165015 Free PMC article.
-
Substrate specificity of 2-deoxy-D-ribose 5-phosphate aldolase (DERA) assessed by different protein engineering and machine learning methods.Appl Microbiol Biotechnol. 2020 Dec;104(24):10515-10529. doi: 10.1007/s00253-020-10960-x. Epub 2020 Nov 4. Appl Microbiol Biotechnol. 2020. PMID: 33147349 Free PMC article.
-
Current state of and need for enzyme engineering of 2-deoxy-D-ribose 5-phosphate aldolases and its impact.Appl Microbiol Biotechnol. 2021 Aug;105(16-17):6215-6228. doi: 10.1007/s00253-021-11462-0. Epub 2021 Aug 19. Appl Microbiol Biotechnol. 2021. PMID: 34410440 Free PMC article. Review.
-
Conformational Sampling of the Intrinsically Disordered C-Terminal Tail of DERA Is Important for Enzyme Catalysis.ACS Catal. 2018 May 4;8(5):3971-3984. doi: 10.1021/acscatal.7b04408. Epub 2018 Mar 27. ACS Catal. 2018. PMID: 30101036 Free PMC article.
-
Exploiting Enzymatic Dynamic Reductive Kinetic Resolution (DYRKR) in Stereocontrolled Synthesis.Adv Synth Catal. 2015 May 26;357(8):1619-1632. doi: 10.1002/adsc.201500316. Epub 2015 May 12. Adv Synth Catal. 2015. PMID: 26622223 Free PMC article.
References
-
- Tobert JA (2003) Lovastatin and beyond: the history of the HMG-COA reductase inhibitors. Nat Rev Drug Discov 2: 517–526. - PubMed
-
- Istvan ES, Deisenhofer J (2001) Structural mechanism for statin inhibition of HMG-CoA reductase. Science 292: 1160–1164. - PubMed
-
- Brautbar A, Ballantyne CM (2011) Pharmacological strategies for lowering LDL cholesterol: statins and beyond. Nat Rev Cardiol 8: 253–265. - PubMed
-
- Kidd J (2006) Life after statin patent expires. Nat Rev Drug Discov 5: 813–814. - PubMed
-
- Almuti K, Rimawi R, Spevack D, Ostfeld RJ (2006) Effects of statins beyond lipid lowering: potential for clinical benefits. Int J Cardiol 109: 7–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

