Adoptive cellular immunotherapy in metastatic renal cell carcinoma: a systematic review and meta-analysis
- PMID: 23667530
- PMCID: PMC3647060
- DOI: 10.1371/journal.pone.0062847
Adoptive cellular immunotherapy in metastatic renal cell carcinoma: a systematic review and meta-analysis
Abstract
Purpose: Metastatic renal cell carcinoma (mRCC), as one of the most immunogenic tumors has been the focus of adoptive cellular immunotherapy (ACI), but the effects of ACI on objective response and survival in patients with mRCC are still controversial. Therefore, a systematic review and meta-analysis was performed to address this issue.
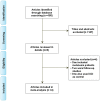
Methods: A search was conducted in the PubMed database for randomized clinical trials (RCTs) with ACI in mRCC. All included articles in this study were assessed according to the selection criteria and were divided into two groups: ACI versus no ACI. Outcomes were toxicity, objective response, 1-, 3- and 5-year survival. Risk ratio (RR) and 95% confidence intervals (CI) were calculated using a fixed-effects meta-analysis. Heterogeneity was measured by value of I(2) or P.
Results: 4 studies (469 patients) were included. Most of ACI-related adverse reactions were grade 1 or 2 and reversible. ACI provided significant benefit in terms of objective response (RR = 1.65; 95% CI, 1.15 to 2.38; P = 0.007, I(2) = 49%), 1-year survival (RR = 1.30; 95% CI, 1.12 to 1.52; P = 0.0008, I(2) = 0%), 3-year survival (RR = 2.76; 95% CI, 1.85 to 4.14; P<0.00001, I(2) = 46%) and 5-year survival (RR = 2.42; 95% CI, 1.21 to 4.83; P = 0.01, I(2) = 28%).
Conclusions: ACI may be a safe and effective treatment for improving objective response, 1-, 3- and 5-year survival in patients with mRCC. Besides, five obstacles for ACI, including high degree of personalization, unsuitable WHO/RECIST response criteria, inadequate identification of tumor-associated antigens (TAAs), lack of effective combination treatments and less attention paid to the quality of ACI products, should be overcome during the successful development of more potent ACI for cancer in the future.
Conflict of interest statement
Figures
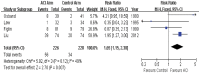



References
-
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363: 411–422. - PubMed
-
- Okur FV, Brenner MK, et al... (2010) Cellular immunotherapy of cancer. In: Yotnda P, editor. Immunotherapy of Cancer: Methods and Protocols. New York: Humana Press/Springer Science+Business Media. 319–345.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

