Engineering T cell function using chimeric antigen receptors identified using a DNA library approach
- PMID: 23667569
- PMCID: PMC3646939
- DOI: 10.1371/journal.pone.0063037
Engineering T cell function using chimeric antigen receptors identified using a DNA library approach
Abstract
Genetic engineering of cellular function holds much promise for the treatment of a variety of diseases including gene deficiencies and cancer. However, engineering the full complement of cellular functions can be a daunting genetic exercise since many molecular triggers need to be activated to achieve complete function. In the case of T cells, genes encoding chimeric antigen receptors (CARs) covalently linking antibodies to cytoplasmic signaling domains can trigger some, but not all, cellular functions against cancer cells. To date, relatively few CAR formats have been investigated using a candidate molecule approach, in which rationally chosen molecules were trialed one by one. Therefore, to expedite this arduous process we developed an innovative screening method to screen many thousands of CAR formats to identify genes able to enhance the anticancer ability of T cells. We used a directional in-frame library of randomly assembled signaling domains in a CAR specific for the tumor associated antigen erbB2. Several new and original CARs were identified, one of which had an enhanced ability to lyse cancer cells and inhibit tumor growth in mice. This study highlights novel technology that could be used to screen a variety of molecules for their capacity to induce diverse functions in cells.
Conflict of interest statement
Figures

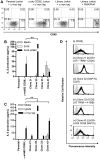

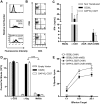

References
-
- Gilham DE, O’Neil A, Hughes C, Guest RD, Kirillova N, et al. (2002) Primary polyclonal human T lymphocytes targeted to carcino-embryonic antigens and neural cell adhesion molecule tumor antigens by CD3zeta-based chimeric immune receptors. J Immunother 25: 139–151. - PubMed
-
- Bridgeman JS, Hawkins RE, Hombach AA, Abken H, Gilham DE (2010) Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther 10: 77–90. - PubMed
-
- Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M (2002) Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol 20: 70–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

