Inhaled steroids modulate extracellular matrix composition in bronchial biopsies of COPD patients: a randomized, controlled trial
- PMID: 23667615
- PMCID: PMC3646783
- DOI: 10.1371/journal.pone.0063430
Inhaled steroids modulate extracellular matrix composition in bronchial biopsies of COPD patients: a randomized, controlled trial
Abstract
Rationale: Smoking and inflammation contribute to the pathogenesis of chronic obstructive pulmonary disease (COPD), which involves changes in extracellular matrix. This is thought to contribute to airway remodeling and airflow obstruction. We have previously observed that long-term treatment with inhaled corticosteroids can not only reduce bronchial inflammation, but can also attenuate lung function decline in moderate-severe COPD. We hypothesized that inhaled corticosteroids and current smoking modulate bronchial extracellular matrix components in COPD.
Objective: To compare major extracellular matrix components (elastic fibers; proteoglycans [versican, decorin]; collagens type I and III) in bronchial biopsies 1) after 30-months inhaled steroids treatment or placebo; and 2) between current and ex-smokers with COPD.
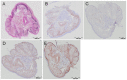
Methods: We included 64 moderate-severe, steroid-naive COPD patients (24/40 (ex)-smokers, 62±7 years, 46 (31-54) packyears, post-bronchodilator forced expiratory volume in one second (FEV1) 62±9% predicted) at baseline in this randomized, controlled trial. 19 and 13 patients received 30-months treatment with fluticasone or placebo, respectively. Bronchial biopsies collected at baseline and after 30 months were studied using (immuno)histochemistry to evaluate extracellular matrix content. Percentage and density of stained area were calculated by digital image analysis.
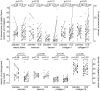
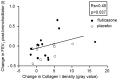
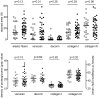
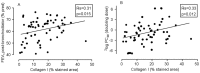
Results: 30-Months inhaled steroids increased the percentage stained area of versican (9.6% [CI 0.9 to 18.3%]; p = 0.03) and collagen III (20.6% [CI 3.8 to 37.4%]; p = 0.02) compared to placebo. Increased collagen I staining density correlated with increased post-bronchodilator FEV1 after inhaled steroids treatment (Rs = 0.45, p = 0.04). There were no differences between smokers and ex-smokers with COPD in percentages and densities for all extracellular matrix proteins.
Conclusions: These data show that long-term inhaled corticosteroids treatment partially changes the composition of extracellular matrix in moderate-severe COPD. This is associated with increased lung function, suggesting that long-term inhaled steroids modulate airway remodeling thereby potentially preventing airway collapse in COPD. Smoking status is not associated with bronchial extracellular matrix proteins.
Trial registration: ClinicalTrials.gov NCT00158847.
Conflict of interest statement
Figures
References
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, et al. (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176: 532–555. - PubMed
-
- Hogg JC, Timens W (2009) The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 4: 435–459. - PubMed
-
- Fernandes DJ, Bonacci JV, Stewart AG (2006) Extracellular matrix, integrins, and mesenchymal cell function in the airways. Curr Drug Targets 7: 567–577. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

