Comparison of rapid diagnostic tests for the detection of Plasmodium vivax malaria in South Korea
- PMID: 23667710
- PMCID: PMC3646812
- DOI: 10.1371/journal.pone.0064353
Comparison of rapid diagnostic tests for the detection of Plasmodium vivax malaria in South Korea
Abstract
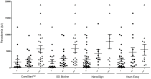
South Korea is one of many countries with endemic Plasmodium vivax malaria. Here we report the evaluation of four rapid diagnostic tests (RDTs) for diagnosis of this disease. A total of 253 subjects were enrolled in the study. The sensitivities, specificities and agreement frequencies were estimated by comparing the four RDTs against the standard of nested-PCR and microscopic examination. The CareStart(TM) and SD Bioline had higher test sensitivities (99.4 and 98.8%, respectively) compared with the NanoSign and Asan Easy tests (93.0 and 94.7%, respectively). The CareStart(TM) and SD Bioline tests could detect P. vivax in samples with parasite densities <150/μl, which was a slightly better performance than the other two RDTs. The quantitative accuracy of the four RDTs was also estimated by comparing results with P. vivax counts from blood samples. Lower test price would result in increased use of these RDTs in the field. The results of this study contribute valuable information that will aid in the selection of a diagnostic method for the detection of malaria.
Conflict of interest statement
Figures

References
-
- Mendis K, Sina BJ, Marchesini P, Carter R (2001) The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 64: 97–106. - PubMed
-
- Bruce-Chwatt LJ (1987) From Laveran's discovery to DNA probes: new trends in diagnosis of malaria. Lancet 2: 1509–1511. - PubMed
-
- Coleman RE, Maneechai N, Rachaphaew N, Kumpitak C, Miller RS, et al. (2002) Comparison of field and expert laboratory microscopy for active surveillance for asymptomatic Plasmodium falciparum and Plasmodium vivax in western Thailand. Am J Trop Med Hyg 67: 141–144. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

