Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials
- PMID: 23668322
- PMCID: PMC3700371
- DOI: 10.1021/nn305872d
Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials
Abstract
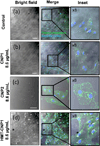
The study of the chemical and biological properties of CeO2 nanoparticles (CNPs) has expanded recently due to its therapeutic potential, and the methods used to synthesize these materials are diverse. Moreover, conflicting reports exist regarding the toxicity of CNPs. To help resolve these discrepancies, we must first determine whether CNPs made by different methods are similar or different in their physicochemical and catalytic properties. In this paper, we have synthesized several forms of CNPs using identical precursors through a wet chemical process but using different oxidizer/reducer; H2O2 (CNP1), NH4OH (CNP2), or hexamethylenetetramine (HMT-CNP1). Physicochemical properties of these CNPs were extensively studied and found to be different depending on the preparation methods. Unlike CNP1 and CNP2, HMT-CNP1 was readily taken into endothelial cells and the aggregation can be visualized using light microscopy. Exposure to HMT-CNP1 also reduced cell viability at a 10-fold lower concentration than CNP1 or CNP2. Surprisingly, exposure to HMT-CNP1 led to substantial decreases in ATP levels. Mechanistic studies revealed that HMT-CNP1 exhibited substantial ATPase (phosphatase) activity. Though CNP2 also exhibits ATPase activity, CNP1 lacked ATPase activity. The difference in catalytic (ATPase) activity of different CNPs preparation may be due to differences in their morphology and oxygen extraction energy. These results suggest that the combination of increased uptake and ATPase activity of HMT-CNP1 may underlie the biomechanism of the toxicity of this preparation of CNPs and may suggest that ATPase activity should be considered when synthesizing CNPs for use in biomedical applications.
Figures







References
-
- Campbell CT, Peden CH. Chemistry. Oxygen Vacancies and Catalysis on Ceria Surfaces. Science. 2005;309:713–714. - PubMed
-
- Tsai YY, Oca-Cossio J, Lin SM, Woan K, Yu PC, Sigmund W. Reactive Oxygen Species Scavenging Properties of ZrO2-CeO2 Solid Solution Nanoparticles. Nanomedicine. 2008;3:637–645. - PubMed
-
- Masui T, Ozaki T, Machida K, Adachi G. Preparation of Ceria-Zirconia Sub-Catalysts for Automotive Exhaust Cleaning. J Alloy Compd. 2000;303:49–55.
-
- Dowding JM, Dosani T, Kumar A, Seal S, Self WT. Cerium Oxide Nanoparticles Scavenge Nitric Oxide Radical (·NO) Chem Commun. 2012;48:4896–4898. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

