In vitro evaluation of anti-infective activity of a Lactobacillus plantarum strain against Salmonella enterica serovar Enteritidis
- PMID: 23668384
- PMCID: PMC3662602
- DOI: 10.1186/1757-4749-5-11
In vitro evaluation of anti-infective activity of a Lactobacillus plantarum strain against Salmonella enterica serovar Enteritidis
Abstract
Background: Salmonella enterica serovar Enteritidis infections are known to exhibit worldwide prevalence with increased morbidity and mortality. The conventional strategies like antibiotic therapy and vaccination have not only proved to be of sub-optimal efficacy but also led to the development of multidrug resistant strains of Salmonella. Antimicrobial activities of probiotics against various enteropathogens and other health promoting effects have assumed greater significance in recent years. The present study aims to evaluate the efficacy of a Lactobacillus plantarum strain (KSBT 56, isolated from a traditional food product of India), in preventing Salmonella enterica serovar Enteritidis growth and pathogenicity in vitro.
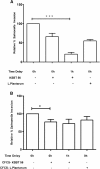
Methods and results: The cell free culture supernatant (CFCS) of KSBT 56 strain notably inhibited the growth of Salmonella Enteritidis without affecting the growth of other gram-positive lactic acid bacteria. The isolated KSBT 56 strain produces lactic acid similar to other standard probiotic strains like Lactobacillus plantarum MTCC 1407. The free radical production by KSBT 56 strain was studied by using sodC mutant of S. Enteritidis, which exhibited reduced growth in the presence of CFCS of the KSBT 56 strain, indicating the inhibitory activity of free radicals on the growth of S. Enteritidis. Our results also showed a significant reduction in the biofilm forming ability of Salmonella Enteritidis in the presence of the KSBT 56 strain (2 log cfu/ml, p = 0.01). Further, the anti-infective characteristics of KSBT 56 strain was validated by gentamicin protection assay which revealed 80% reduction in the invasion of Salmonella Enteritidis to HCT-116 cell line (Salmonella Enteritidis and KSBT 56 in a 1:1 ratio) and delayed addition of Salmonella Enteritidis by 1 h. Similarly, the reduced adhesion of Salmonella to the HCT-116 cells was observed along with the down regulation of hilA gene of Salmonella Pathogenicity Island 1 (SPI1) indicating that they might have acted synergistically to decrease the invasion of the pathogen into the cell line.
Conclusions: KSBT 56 strain effectively inhibited the growth, invasion and the biofilm forming ability of Salmonella Enteritidis without inhibiting the growth of other Lactobacillus strains. Overall, our result suggested that KSBT 56 can be used as a potential probiotic strain with considerable beneficial effects on the host.
Figures




References
-
- Birosova L, Mikulasova M. Development of triclosan and antibiotic resistance in Salmonella enterica serovar Typhimurium. J Med Microbiol. 2009;58(Pt 4):436–441. - PubMed
-
- Alcaine SD, Warnick LD, Wiedmann M. Antimicrobial resistance in nontyphoidal Salmonella. J Food Prot. 2007;70(3):780–790. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

