Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes
- PMID: 23668478
- PMCID: PMC3906841
- DOI: 10.1111/dom.12127
Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes
Abstract
Aims: Sodium-glucose co-transporter 2 (SGLT2) reabsorbs glucose and sodium in the renal proximal tubule. Dapagliflozin, an SGLT2 inhibitor, targets hyperglycaemia in type 2 diabetes by increasing renal glucose excretion. To investigate whether the parallel occurring sodium loss would have diuretic-like physiologic effects, we compared dapagliflozin and hydrochlorothiazide (HCTZ) effects on 24-h blood pressure (BP), body weight, plasma volume and glomerular filtration rate (GFR).
Methods: In this randomized, placebo-controlled, double-blind trial, 75 subjects with type 2 diabetes were assigned placebo, dapagliflozin 10 mg/day, or HCTZ 25 mg/day. Changes from baseline BP, body weight, plasma volume and GFR were assessed after 12 weeks of treatment.
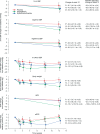
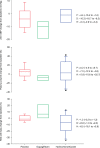
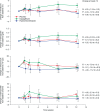
Results: Subjects' mean age was 56 years, type 2 diabetes mellitus (T2DM) duration 6.3 years, and haemoglobin A1c (HbA1c) 7.5%. Treatment with placebo, dapagliflozin or HCTZ resulted in changes from baseline in 24-h ambulatory mean systolic blood pressure (SBP) of -0.9 (95%CI -4.2, +2.4), -3.3 (95%CI -6.8, +0.2), and -6.6 (95%CI -9.9, -3.2) mmHg, respectively at week 12, adjusted for baseline SBP. Body weight decreased with dapagliflozin and HCTZ. In a sub-study plasma volume appeared to decrease with dapagliflozin but did not change with placebo or HCTZ treatment. Dapagliflozin induced a greater reduction in GFR (-10.8%; 95%CI -14.6, -6.7) relative to placebo (-2.9%; 95% CI -6.9, +1.2) or HCTZ (-3.4%; 95%CI -7.3, +0.6).
Conclusions: Dapagliflozin-induced SGLT2 inhibition for 12 weeks is associated with reductions in 24-h BP, body weight, GFR and possibly plasma volume. Cumulatively, these effects suggest that dapagliflozin may have a diuretic-like capacity to lower BP in addition to beneficial effects on glycaemic control.
Keywords: HbA1c; blood pressure; dapagliflozin; renal function; type 2 diabetes.
© 2013 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years.Diabetes Obes Metab. 2014 Feb;16(2):124-36. doi: 10.1111/dom.12187. Epub 2013 Aug 29. Diabetes Obes Metab. 2014. PMID: 23911013 Clinical Trial.
-
[Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride].Dtsch Med Wochenschr. 2013 Apr;138 Suppl 1:S16-26. doi: 10.1055/s-0032-1305277. Epub 2013 Mar 25. Dtsch Med Wochenschr. 2013. PMID: 23529567 Clinical Trial. German.
-
Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study.Lancet Diabetes Endocrinol. 2016 Mar;4(3):211-220. doi: 10.1016/S2213-8587(15)00417-9. Epub 2015 Nov 27. Lancet Diabetes Endocrinol. 2016. PMID: 26620248 Clinical Trial.
-
Impact of sodium-glucose cotransporter 2 inhibitors on blood pressure.Vasc Health Risk Manag. 2016 Oct 27;12:393-405. doi: 10.2147/VHRM.S111991. eCollection 2016. Vasc Health Risk Manag. 2016. PMID: 27822054 Free PMC article. Review.
-
[Dapagliflozin, the first SGLT-2 inhibitor in the treatment of type 2 diabetes].Med Clin (Barc). 2013 Sep;141 Suppl 2:36-43. doi: 10.1016/S0025-7753(13)70062-9. Med Clin (Barc). 2013. PMID: 24444523 Review. Spanish.
Cited by
-
SGLT2 Inhibitor Use and Risk of Breast Cancer Among Adult Women with Type 2 Diabetes.Drug Saf. 2024 Feb;47(2):125-133. doi: 10.1007/s40264-023-01373-6. Epub 2023 Dec 9. Drug Saf. 2024. PMID: 38070101
-
Sodium-dependent glucose transporter 2 inhibitors effects on myocardial function in patients with type 2 diabetes and asymptomatic heart failure.World J Cardiol. 2024 Aug 26;16(8):448-457. doi: 10.4330/wjc.v16.i8.448. World J Cardiol. 2024. PMID: 39221192 Free PMC article.
-
Obesity-related heart failure with preserved ejection fraction: diagnostic and therapeutic challenges.Korean J Intern Med. 2023 Mar;38(2):157-166. doi: 10.3904/kjim.2022.271. Epub 2023 Feb 6. Korean J Intern Med. 2023. PMID: 36740840 Free PMC article. Review.
-
Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Water and Sodium Metabolism.Front Pharmacol. 2022 Feb 23;13:800490. doi: 10.3389/fphar.2022.800490. eCollection 2022. Front Pharmacol. 2022. PMID: 35281930 Free PMC article. Review.
-
Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction.Cardiovasc Diabetol. 2019 Aug 20;18(1):107. doi: 10.1186/s12933-019-0914-1. Cardiovasc Diabetol. 2019. PMID: 31429767 Free PMC article.
References
-
- Feinglos M, Dailey G, Cefalu W, et al. Effect on glycemic control of the addition of 2.5 mg glipizide GITS to metformin in patients with T2DM. Diabetes Res Clin Pract. 2005;68:167–175. - PubMed
-
- Hamann A, Garcia-Puig J, Paul G, Donaldson J, Stewart M. Comparison of fixed-dose rosiglitazone/metformin combination therapy with sulphonylurea plus metformin in overweight individuals with type 2 diabetes inadequately controlled on metformin alone. Exp Clin Endocrinol Diabetes. 2008;116:6–13. - PubMed
-
- Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410–1418. - PubMed
-
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. - PubMed
-
- Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85:520–526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

