Disordered Toll-like receptor 2 responses in the pathogenesis of pulmonary sarcoidosis
- PMID: 23668840
- PMCID: PMC3949639
- DOI: 10.1111/cei.12138
Disordered Toll-like receptor 2 responses in the pathogenesis of pulmonary sarcoidosis
Abstract
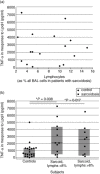
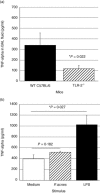
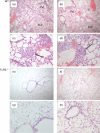
In this study, we hypothesized that the granulomatous disorder sarcoidosis is not caused by a single pathogen, but rather results from abnormal responses of Toll-like receptors (TLRs) to conserved bacterial elements. Unsorted bronchoalveolar lavage (BAL) cells from patients with suspected pulmonary sarcoidosis and healthy non-smoking control subjects were stimulated with representative ligands of TLR-2 (in both TLR-2/1 and TLR-2/6 heterodimers) and TLR-4. Responses were determined by assessing resulting production of tumour necrosis factor (TNF)-α and interleukin (IL)-6. BAL cells from patients in whom sarcoidosis was confirmed displayed increased cytokine responses to the TLR-2/1 ligand 19-kDa lipoprotein of Mycobacterium tuberculosis (LpqH) and decreased responses to the TLR-2/6 agonist fibroblast stimulating ligand-1 (FSL)-1. Subsequently, we evaluated the impact of TLR-2 gene deletion in a recently described murine model of T helper type 1 (Th1)-associated lung disease induced by heat-killed Propionibacterium acnes. As quantified by blinded scoring of lung pathology, P. acnes-induced granulomatous pulmonary inflammation was markedly attenuated in TLR-2(-/-) mice compared to wild-type C57BL/6 animals. The findings support a potential role for disordered TLR-2 responses in the pathogenesis of pulmonary sarcoidosis.
Keywords: Toll-like receptors; bronchoalveolar lavage (BAL); sarcoidosis.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Figures



Similar articles
-
Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2.Am J Respir Crit Care Med. 2010 Feb 15;181(4):360-73. doi: 10.1164/rccm.200905-0696OC. Epub 2009 Nov 12. Am J Respir Crit Care Med. 2010. PMID: 19910611 Free PMC article.
-
Dysregulation of p38 and MKP-1 in response to NOD1/TLR4 stimulation in sarcoid bronchoalveolar cells.Am J Respir Crit Care Med. 2011 Feb 15;183(4):500-10. doi: 10.1164/rccm.201005-0792OC. Epub 2010 Sep 17. Am J Respir Crit Care Med. 2011. PMID: 20851927 Free PMC article.
-
Functional Toll-Like Receptor 9 Expression and CXCR3 Ligand Release in Pulmonary Sarcoidosis.Am J Respir Cell Mol Biol. 2016 Nov;55(5):749-757. doi: 10.1165/rcmb.2015-0278OC. Am J Respir Cell Mol Biol. 2016. PMID: 27390897
-
Burn-induced alterations in toll-like receptor-mediated responses by bronchoalveolar lavage cells.Cytokine. 2011 Sep;55(3):396-401. doi: 10.1016/j.cyto.2011.05.004. Epub 2011 Jun 21. Cytokine. 2011. PMID: 21696980 Free PMC article.
-
Briefing of pulmonary sarcoidosis: Reduction-oxidation, misleading and possibilities.Indian J Tuberc. 2025 Jan;72(1):103-111. doi: 10.1016/j.ijtb.2024.07.003. Epub 2024 Jul 25. Indian J Tuberc. 2025. PMID: 39890360 Review.
Cited by
-
Chronic Inflammation in Immune Aging: Role of Pattern Recognition Receptor Crosstalk with the Telomere Complex?Front Immunol. 2017 Sep 4;8:1078. doi: 10.3389/fimmu.2017.01078. eCollection 2017. Front Immunol. 2017. PMID: 28928745 Free PMC article. Review.
-
Structure-Based Virtual Screening of Benzaldehyde Thiosemicarbazone Derivatives against DNA Gyrase B of Mycobacterium tuberculosis.Evid Based Complement Alternat Med. 2021 Dec 13;2021:6140378. doi: 10.1155/2021/6140378. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34938343 Free PMC article.
-
Sarcoidosis-Related Cardiomyopathy: Current Knowledge, Challenges, and Future Perspectives State-of-the-Art Review.J Card Fail. 2022 Jan;28(1):113-132. doi: 10.1016/j.cardfail.2021.06.016. Epub 2021 Jul 11. J Card Fail. 2022. PMID: 34260889 Free PMC article. Review.
-
Granuloma formation in pulmonary sarcoidosis.Front Immunol. 2013 Dec 10;4:437. doi: 10.3389/fimmu.2013.00437. Front Immunol. 2013. PMID: 24339826 Free PMC article. Review.
-
The composition of the pulmonary microbiota in sarcoidosis - an observational study.Respir Res. 2019 Feb 28;20(1):46. doi: 10.1186/s12931-019-1013-2. Respir Res. 2019. PMID: 30819175 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

