The zebrafish as a model to study polycystic liver disease
- PMID: 23668934
- PMCID: PMC3673589
- DOI: 10.1089/zeb.2012.0825
The zebrafish as a model to study polycystic liver disease
Abstract
In the polycystic liver diseases (PLD), genetic defects initiate the formation of cysts in the liver and kidney. In rodent models of PLD (i.e., the PCK rat and Pkd2(WS25/-) mouse), we have studied hepatorenal cystic disease and therapeutic approaches. In this study, we employed zebrafish injected with morpholinos against genes involved in the PLD, including sec63, prkcsh, and pkd1a. We calculated the liver cystic area, and based on our rodent studies, we exposed the embryos to pasireotide [1 μM] or vitamin K3 [100 μM] and assessed the endoplasmic reticulum (ER) in cholangiocytes in embryos treated with 4-phenylbutyrate (4-PBA). Our results show that (a) morpholinos against sec63, prkcsh, and pkd1a eliminate expression of the respective proteins; (b) phenotypic body changes included curved tail and the formation of hepatic cysts in zebrafish larvae; (c) exposure of embryos to pasireotide inhibited hepatic cystogenesis in the zebrafish models; and (d) exposure of embryos to 4-PBA resulted in the ER in cholangiocytes resolving from a curved to a smooth appearance. Our results suggest that the zebrafish model of PLD may provide a means to screen drugs that could inhibit hepatic cystogenesis.
Figures

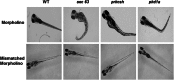
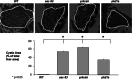
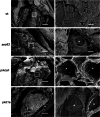


References
-
- Masyuk TV. Masyuk AI. Torres VE. Harris PC. LaRusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

