Cytochrome P450 2E1 potentiates ethanol induction of hypoxia and HIF-1α in vivo
- PMID: 23669278
- PMCID: PMC3729858
- DOI: 10.1016/j.freeradbiomed.2013.05.009
Cytochrome P450 2E1 potentiates ethanol induction of hypoxia and HIF-1α in vivo
Abstract
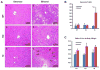
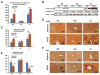
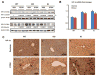
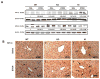
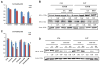
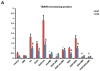
Ethanol induces hypoxia and elevates HIF-1α in the liver. CYP2E1 plays a role in the mechanisms by which ethanol generates oxidative stress, fatty liver, and liver injury. This study evaluated whether CYP2E1 contributes to ethanol-induced hypoxia and activation of HIF-1α in vivo and whether HIF-1α protects against or promotes CYP2E1-dependent toxicity in vitro. Wild-type (WT), CYP2E1-knock-in (KI), and CYP2E1 knockout (KO) mice were fed ethanol chronically; pair-fed controls received isocaloric dextrose. Ethanol produced liver injury in the KI mice to a much greater extent than in the WT and KO mice. Protein levels of HIF-1α and downstream targets of HIF-1α activation were elevated in the ethanol-fed KI mice compared to the WT and KO mice. Levels of HIF prolyl hydroxylase 2, which promotes HIF-1α degradation, were decreased in the ethanol-fed KI mice in association with the increases in HIF-1α. Hypoxia occurred in the ethanol-fed CYP2E1 KI mice as shown by an increased area of staining using the hypoxia-specific marker pimonidazole. Hypoxia was lower in the ethanol-fed WT mice and lowest in the ethanol-fed KO mice and all the dextrose-fed mice. In situ double staining showed that pimonidazole and CYP2E1 were colocalized to the same area of injury in the hepatic centrilobule. Increased protein levels of HIF-1α were also found after acute ethanol treatment of KI mice. Treatment of HepG2 E47 cells, which express CYP2E1, with ethanol plus arachidonic acid (AA) or ethanol plus buthionine sulfoximine (BSO), which depletes glutathione, caused loss of cell viability to a greater extent than in HepG2 C34 cells, which do not express CYP2E1. These treatments elevated protein levels of HIF-1α to a greater extent in E47 cells than in C34 cells. 2-Methoxyestradiol, an inhibitor of HIF-1α, blunted the toxic effects of ethanol plus AA and ethanol plus BSO in the E47 cells in association with inhibition of HIF-1α. The HIF-1α inhibitor also blocked the elevated oxidative stress produced by ethanol/AA or ethanol/BSO in the E47 cells. These results suggest that CYP2E1 plays a role in ethanol-induced hypoxia, oxidative stress, and activation of HIF-1α and that HIF-1α contributes to CYP2E1-dependent ethanol-induced toxicity. Blocking HIF-1α activation and actions may have therapeutic implications for protection against ethanol/CYP2E1-induced oxidative stress, steatosis, and liver injury.
Keywords: 2-ME; 2-methoxyestradiol; 3-NT; 3-nitrotyrosine; 4-HNE; 4-hydroxynonenal; AA; ALT; BSO; CYP2E1; Ethanol; Free radicals; GSH; HE; HIF; HIF prolyl hydroxylase 2; HIF-1α; HPH-2; Hepatotoxicity; IHC; KI; KO; LDHA; MDA; Oxidative stress; PNP; ROS; TBARS; WT; alanine aminotransferase; arachidonic acid; cytochrome P450 2E1; hematoxylin–eosin; hypoxia-inducible factor; immunohistochemistry; knock-in; knockout; l-buthionine sulfoximine; lactate dehydrogenase A; malondialdehyde; p-nitrophenol; reactive oxygen species; reduced glutathione; thiobarbituric acid-reactive substances; wild type.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Semenza GL. Regulation of mammalian oxygen homeostasis by hypoxia- inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. - PubMed
-
- Formenti F, Constantin-Tedusoiv D, Emmanuel Y, Cheeseman J, Durrington KL, Edwards LM, Humphreys SM, Lappin TR, McMullin MF, McNamara CJ, Mills W, Murphy JA, O’Connor DF, Percy MJ, Ratcliffe PJ, Smith TG, Treacy M, Frayn KN, Greenhaff PL, Karpe F, Clarke K, Robbins PA. Regulation of human metabolism by hypoxia-inducible factor. Proc Natl Acad Sci USA. 2010;107:12722–12727. - PMC - PubMed
-
- Juan M, Kondo K, Yang H, Kim W, Valiando J, Ohn M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for oxygen sensing. Science. 2001;292:464–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

