Uptake of genetic testing by relatives of lynch syndrome probands: a systematic review
- PMID: 23669308
- PMCID: PMC12685307
- DOI: 10.1016/j.cgh.2013.04.044
Uptake of genetic testing by relatives of lynch syndrome probands: a systematic review
Abstract
Background & aims: Screening of persons with newly diagnosed colorectal cancer for Lynch syndrome can yield substantial benefits at acceptable costs, presuming sufficient uptake of genetic testing by first-degree relatives of Lynch syndrome probands. We performed a systematic review of the literature to determine the frequency of and factors associated with genetic testing of first-degree relatives of Lynch syndrome probands.
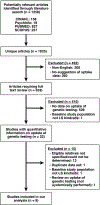
Methods: We searched 4 databases (CINAHL, PsycInfo, PUBMED, and SCOPUS) for articles published through May 2011 reporting uptake of genetic testing by relatives of Lynch syndrome probands. Two investigators independently screened articles to determine whether they met inclusion criteria; data were collected on study population, genetic counseling, and genetic testing. A narrative, qualitative systematic review was performed.
Results: We identified 1258 potentially relevant articles; 533 underwent full-text review, and 8 were included in the final analysis. Of first-degree relatives of Lynch syndrome probands, 52% or less received genetic testing. For each proband, 3.6 or fewer relatives underwent genetic testing. Demographic factors (age <50 years, female sex, parenthood, level of education, employment, participation in medical studies), psychological factors (lack of depressive symptoms), and possibly family history (greater number of relatives with cancer) were associated with uptake of genetic testing.
Conclusions: Genetic testing appears to be underutilized by first-degree relatives of patients with Lynch syndrome. The clinical benefit and economic feasibility of screening persons with colorectal cancer for Lynch syndrome depend on optimizing family-wide uptake of genetic testing. Future research and clinical efforts should focus on ways to overcome barriers to genetic testing.
Keywords: CI; CRC; Colorectal Cancer; FDR; First-degree Relatives; GINA; Genetic Information Nondiscrimination Act; Genetic Testing; LS; Lynch Syndrome; Lynch syndrome; NOS; Newcastle–Ottawa Scale; OR; Second-degree Relative; colorectal cancer; confidence interval; first-degree relative; odds ratio.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
Colorectal cancer: Cascade genetic testing in Lynch syndrome: room for improvement.Nat Rev Gastroenterol Hepatol. 2013 Sep;10(9):506-8. doi: 10.1038/nrgastro.2013.122. Epub 2013 Jul 9. Nat Rev Gastroenterol Hepatol. 2013. PMID: 23835491
References
-
- Burt R, Barthel J, Dunn K, David D, Drelichman E, Ford J, Giardiello F, Gruber S, Halverson A, Hamilton S, Ismail M, Jasperson K, Lazenby A, Lynch P, Martin E Jr, Mayer R, Ness R, Provenzale D, Rao M, Shike M, Steinbach G, Terdiman J, Weinberg D. Colorectal cancer screening: Clinical practice guidelines in oncology™. JNCCN Journal of the National Comprehensive Cancer Network 2010;8:8–60. - PubMed
-
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous