The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement
- PMID: 23669423
- PMCID: PMC4844002
- DOI: 10.1158/1078-0432.CCR-12-2935
The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement
Abstract
In selecting an endpoint in clinical trial design, it is important to consider that the endpoint is both reliably measured and clinically meaningful. As such, overall survival (OS) has traditionally been considered the most clinically relevant and convincing endpoint in clinical trial design as long as it is accompanied by preservation in quality of life. However, progression-free survival (PFS) is increasingly more prominent in clinical trial design because of feasibility issues (smaller sample sizes and shorter follow-up). PFS has the advantage of taking into account not only responsive disease, but stable disease as well, an issue of particular importance in the relapsed and refractory setting in which therapies are often associated with a minimal to nil response but may still confer a survival advantage. Finally, PFS has a significant advantage in molecularly selected populations, in whom OS advantages are difficult to detect due to the effects of crossover. With an understanding of the limitations and biases that are introduced with PFS as a primary endpoint, we believe that PFS is not only a viable but also a necessary alternative to OS in assessing the efficacy of selected novel-targeted therapies in molecularly defined cancer populations. Ultimately, the selection of a clinical trial endpoint should not be based on a one-size-fits all approach; rather, it should be based on the specifics of the therapeutic strategy being tested and the population under study.
©2013 AACR
Conflict of interest statement
No potential conflicts of interests were disclosed.
Figures

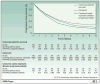

References
-
- Moertel CG, Hanley JA. The effect of measuring error on the results of therapeutic trials in advanced cancer. Cancer. 1976;38:388–94. - PubMed
-
- Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much? Clin Cancer Res. 2010;16:5972–80. - PubMed
-
- Wilkerson J, Fojo T. Progression-free survival is simply a measure of a drug’s effect while administered and is not a surrogate for overall survival. Cancer J. 2009;15:379–85. - PubMed
-
- U.S. Food and Drug Administration. Clinical trial endpoints for the approval of cancer drugs and biologics. Rockville, MD: U.S. Department of Health and Human Services; 2007. Guidance for industry.
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

