Replacement of the C6ORF66 assembly factor (NDUFAF4) restores complex I activity in patient cells
- PMID: 23670274
- PMCID: PMC3745599
- DOI: 10.2119/molmed.2012.00343
Replacement of the C6ORF66 assembly factor (NDUFAF4) restores complex I activity in patient cells
Abstract
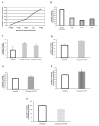
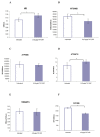
Disorders of the oxidative phosphorylation (OXPHOS) system frequently result in a severe multisystem disease with the consequence of early childhood death. Among these disorders, isolated complex I deficiency is the most frequently diagnosed, accounting for one-third of all cases of respiratory chain deficiency. We chose to focus on complex I deficiency, caused by mutation in the assembly factor chromosome 6, open reading frame 66 (C6ORF66; NADH dehydrogenase [ubiquinone] complex I assembly factor 4 [NDUFAF4]) protein. We used the approach of cell- and organelle-directed protein/enzyme replacement therapy, with the transactivator of transcription (TAT) peptide as the moiety delivery system. This step will enable us to deliver the wild-type assembly factor C6ORF66 into patient cells and their mitochondria, leading to the proper assembly and function of complex I and, as a result, to a functional OXPHOS system. We designed and constructed the TAT-ORF fusion protein by gene fusion techniques, expressed the protein in an Escherichia coli expression system and highly purified it. Our results indicate that TAT-ORF enters patients' cells and their mitochondria rapidly and efficiently. TAT-ORF is biologically active and led to an increase in complex I activity. TAT-ORF also increased the number of patient cells and improved the activity of their mitochondria. Moreover, we observed an increase in ATP production, a decrease in the content of mitochondria and a decrease in the level of reactive oxygen species. Our results suggest that this approach of protein replacement therapy for the treatment of mitochondrial disorders is a promising one.
Figures




References
-
- Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N Engl J Med. 2012;366:1132–41. - PubMed
-
- Thorburn DR. Mitochondrial disorders: prevalence, myths and advances. J Inherit Metab Dis. 2004;27:349–62. - PubMed
-
- Balsa E, et al. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 2012;16:378–86. - PubMed
-
- Janssen R, Smeitink J, Smeets R, van Den Heuvel L. CIA30 complex I assembly factor: a candidate for human complex I deficiency? Hum Genet. 2002;110:264–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

