Development of a porous poly(DL-lactic acid-co-glycolic acid)-based scaffold for mastoid air-cell regeneration
- PMID: 23670365
- PMCID: PMC4501022
- DOI: 10.1002/lary.24173
Development of a porous poly(DL-lactic acid-co-glycolic acid)-based scaffold for mastoid air-cell regeneration
Abstract
Objectives/hypothesis: To develop a porous, biodegradable scaffold for mastoid air-cell regeneration.
Study design: In vitro development of a temperature-sensitive poly(DL-lactic acid-co-glycolic acid)/poly(ethylene glycol) (PLGA/PEG) scaffold tailored for this application.
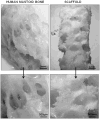
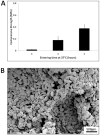
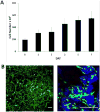
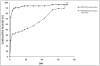
Methods: Human mastoid bone microstructure and porosity were investigated using micro-computed tomography. PLGA/PEG-alginate scaffolds were developed, and scaffold porosity was assessed. Human bone marrow mesenchymal stem cells (hBM-MSCs) were cultured on the scaffolds in vitro. Scaffolds were loaded with ciprofloxacin, and release of ciprofloxacin over time in vitro was assessed.
Results: Porosity of human mastoid bone was measured at 83% with an average pore size of 1.3 mm. PLGA/PEG-alginate scaffold porosity ranged from 43% to 78% depending on the alginate bead content. The hBM-MSCs proliferate on the scaffolds in vitro, and release of ciprofloxacin from the scaffolds was demonstrated over 7 to 10 weeks.
Conclusions: The PLGA/PEG-alginate scaffolds developed in this study demonstrate similar structural features to human mastoid bone, support cell growth, and display sustained antibiotic release. These scaffolds may be of potential clinical use in mastoid air-cell regeneration. Further in vivo studies to assess the suitability of PLGA/PEG-alginate scaffolds for this application are required.
Keywords: Scaffold; alginate; ciprofloxacin; mastoid; poly(DL-lactic acid-co-glycolic acid).
© 2013 The Authors. The Laryngoscope is published by Wiley Periodicals, Inc. on behalf of The American Laryngological, Rhinological and Otological Society, Inc.
Conflict of interest statement
Figures


References
-
- Cinamon U, Sade J. Mastoid and tympanic membrane as pressure buffers: a quantitative study in a middle ear cleft model. Otol Neurotol. 2003 Nov;24(6):839–842. - PubMed
-
- Doyle WJ, Alper CM, Banks JM, Swarts JD. Rate of nitrous oxide exchange across the middle ear mucosa in monkeys before and after blockage of the mastoid antrum. Otolaryngol Head Neck Surg. 2003 May;128(5):732–741. - PubMed
-
- Kanemaru S-i, Nakamura T, Omori K, Magrufov A, Yamashita M, Ito J. Regeneration of Mastoid Air Cells in Clinical Applications by In Situ Tissue Engineering. The Laryngoscope. 2005;115(2):253–258. - PubMed
-
- Dhillon A, Schneider P, Kuhn G, et al. Analysis of sintered polymer scaffolds using concomitant synchrotron computed tomography and in situ mechanical testing. J Mater Sci Mater Med. 2011 Dec;22(12):2599–2605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

