Changes in helical content or net charge of apolipoprotein C-I alter its affinity for lipid/water interfaces
- PMID: 23670531
- PMCID: PMC3679394
- DOI: 10.1194/jlr.M037531
Changes in helical content or net charge of apolipoprotein C-I alter its affinity for lipid/water interfaces
Abstract
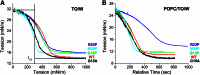
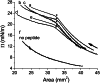
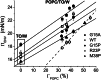
Amphipathic α-helices mediate binding of exchangeable apolipoproteins to lipoproteins. To probe the role of α-helical structure in protein-lipid interactions, we used oil-drop tensiometry to characterize the interfacial behavior of apolipoprotein C-I (apoC-I) variants at triolein/water (TO/W) and 1-palmitoyl-2-oleoylphosphatidylcholine/triolein/water (POPC/TO/W) interfaces. ApoC-I, the smallest apolipoprotein, has two amphipathic α-helices. Mutants had single Pro or Ala substitutions that resulted in large differences in helical content in solution and on phospholipids. The ability of apoC-I to bind TO/W and POPC/TO/W interfaces correlated strongly with α-helical propensity. On binding these interfaces, peptides with higher helical propensity increased surface pressure to a greater extent. Likewise, peptide exclusion pressure at POPC/TO/W interfaces increased with greater helical propensity. ApoC-I retention on TO/W and POPC/TO/W interfaces correlated strongly with phospholipid-bound helical content. On compression of these interfaces, peptides with higher helical content were ejected at higher pressures. Substitution of Arg for Pro in the N-terminal α-helix altered net charge and reduced apoC-I affinity for POPC/TO/W interfaces. Our results suggest that peptide-lipid interactions drive α-helix binding to and retention on lipoproteins. Point mutations in small apolipoproteins could significantly change α-helical propensity or charge, thereby disrupting protein-lipid interactions and preventing the proteins from regulating lipoprotein catabolism at high surface pressures.
Keywords: drop tensiometry; protein-lipid interaction; surface chemistry.
Figures




Similar articles
-
Apolipoprotein C-I binds more strongly to phospholipid/triolein/water than triolein/water interfaces: a possible model for inhibiting cholesterol ester transfer protein activity and triacylglycerol-rich lipoprotein uptake.Biochemistry. 2012 Feb 14;51(6):1238-48. doi: 10.1021/bi2015212. Epub 2012 Feb 2. Biochemistry. 2012. PMID: 22264166 Free PMC article.
-
Surface tensiometry of apolipoprotein B domains at lipid interfaces suggests a new model for the initial steps in triglyceride-rich lipoprotein assembly.J Biol Chem. 2014 Mar 28;289(13):9000-12. doi: 10.1074/jbc.M113.540955. Epub 2014 Feb 10. J Biol Chem. 2014. PMID: 24515109 Free PMC article.
-
The interfacial properties of ApoA-I and an amphipathic alpha-helix consensus peptide of exchangeable apolipoproteins at the triolein/water interface.J Biol Chem. 2005 Feb 11;280(6):4154-65. doi: 10.1074/bc.M411618200. J Biol Chem. 2005. PMID: 15695525
-
Apolipoprotein/lipid interactions: studies with synthetic polypeptides.CRC Crit Rev Biochem. 1982;13(1):87-107. doi: 10.3109/10409238209108710. CRC Crit Rev Biochem. 1982. PMID: 6813024 Review.
-
Lipid-apolipoprotein interactions in amyloid fibril formation and relevance to atherosclerosis.Biochim Biophys Acta Proteins Proteom. 2019 May;1867(5):502-507. doi: 10.1016/j.bbapap.2018.08.010. Epub 2018 Sep 1. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 35818279 Review.
Cited by
-
A Pressure-dependent Model for the Regulation of Lipoprotein Lipase by Apolipoprotein C-II.J Biol Chem. 2015 Jul 17;290(29):18029-18044. doi: 10.1074/jbc.M114.629865. Epub 2015 May 29. J Biol Chem. 2015. PMID: 26026161 Free PMC article.
-
Integrated Quantitative Targeted Lipidomics and Proteomics Reveal Unique Fingerprints of Multiple Metabolic Conditions.Biomolecules. 2022 Oct 8;12(10):1439. doi: 10.3390/biom12101439. Biomolecules. 2022. PMID: 36291648 Free PMC article.
-
Surface behavior of apolipoprotein A-I and its deletion mutants at model lipoprotein interfaces.J Lipid Res. 2014 Mar;55(3):478-92. doi: 10.1194/jlr.M044743. Epub 2013 Dec 5. J Lipid Res. 2014. PMID: 24308948 Free PMC article.
-
Apolipoprotein C1: Its Pleiotropic Effects in Lipid Metabolism and Beyond.Int J Mol Sci. 2019 Nov 26;20(23):5939. doi: 10.3390/ijms20235939. Int J Mol Sci. 2019. PMID: 31779116 Free PMC article. Review.
-
Strategic optimization of conditions for the solubilization of GST-tagged amphipathic helix-containing ciliary proteins overexpressed as inclusion bodies in E. coli.Microb Cell Fact. 2022 Dec 12;21(1):258. doi: 10.1186/s12934-022-01979-y. Microb Cell Fact. 2022. PMID: 36510188 Free PMC article.
References
-
- Mahley R. W., Innerarity T. L., Rall S. C., Weisgraber K. H. 1984. Plasma lipoproteins: apolipoprotein structure and function. J. Lipid Res. 25: 1277–1294 - PubMed
-
- Atkinson D., Small D. M. 1986. Recombinant lipoproteins: implications for structure and assembly of native lipoproteins. Annu. Rev. Biophys. Biophys. Chem. 15: 403–456 - PubMed
-
- von Eckardstein A., Nofer J. R., Assmann G. 2001. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 21: 13–27 - PubMed
-
- Kottke B. A., Zinsmeister A. R., Holmes M. D., Jr, Kneller R. W., Hallaway B. J., Mao S. J. 1986. Apolipoproteins and coronary artery disease. Mayo Clin. Proc. 61: 313–320 - PubMed
-
- Albers J. J., Lin J. T., Roberts G. P. 1979. Effect of human plasma apolipoproteins on the activity of purified lecithin:cholesterol acyltransferase. Artery. 5: 61–75 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

