Changes in helical content or net charge of apolipoprotein C-I alter its affinity for lipid/water interfaces
- PMID: 23670531
- PMCID: PMC3679394
- DOI: 10.1194/jlr.M037531
Changes in helical content or net charge of apolipoprotein C-I alter its affinity for lipid/water interfaces
Abstract
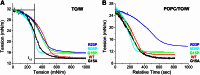
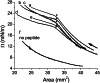
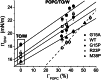
Amphipathic α-helices mediate binding of exchangeable apolipoproteins to lipoproteins. To probe the role of α-helical structure in protein-lipid interactions, we used oil-drop tensiometry to characterize the interfacial behavior of apolipoprotein C-I (apoC-I) variants at triolein/water (TO/W) and 1-palmitoyl-2-oleoylphosphatidylcholine/triolein/water (POPC/TO/W) interfaces. ApoC-I, the smallest apolipoprotein, has two amphipathic α-helices. Mutants had single Pro or Ala substitutions that resulted in large differences in helical content in solution and on phospholipids. The ability of apoC-I to bind TO/W and POPC/TO/W interfaces correlated strongly with α-helical propensity. On binding these interfaces, peptides with higher helical propensity increased surface pressure to a greater extent. Likewise, peptide exclusion pressure at POPC/TO/W interfaces increased with greater helical propensity. ApoC-I retention on TO/W and POPC/TO/W interfaces correlated strongly with phospholipid-bound helical content. On compression of these interfaces, peptides with higher helical content were ejected at higher pressures. Substitution of Arg for Pro in the N-terminal α-helix altered net charge and reduced apoC-I affinity for POPC/TO/W interfaces. Our results suggest that peptide-lipid interactions drive α-helix binding to and retention on lipoproteins. Point mutations in small apolipoproteins could significantly change α-helical propensity or charge, thereby disrupting protein-lipid interactions and preventing the proteins from regulating lipoprotein catabolism at high surface pressures.
Keywords: drop tensiometry; protein-lipid interaction; surface chemistry.
Figures




References
-
- Mahley R. W., Innerarity T. L., Rall S. C., Weisgraber K. H. 1984. Plasma lipoproteins: apolipoprotein structure and function. J. Lipid Res. 25: 1277–1294 - PubMed
-
- Atkinson D., Small D. M. 1986. Recombinant lipoproteins: implications for structure and assembly of native lipoproteins. Annu. Rev. Biophys. Biophys. Chem. 15: 403–456 - PubMed
-
- von Eckardstein A., Nofer J. R., Assmann G. 2001. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 21: 13–27 - PubMed
-
- Kottke B. A., Zinsmeister A. R., Holmes M. D., Jr, Kneller R. W., Hallaway B. J., Mao S. J. 1986. Apolipoproteins and coronary artery disease. Mayo Clin. Proc. 61: 313–320 - PubMed
-
- Albers J. J., Lin J. T., Roberts G. P. 1979. Effect of human plasma apolipoproteins on the activity of purified lecithin:cholesterol acyltransferase. Artery. 5: 61–75 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

