A novel vitreous substitute of using a foldable capsular vitreous body injected with polyvinylalcohol hydrogel
- PMID: 23670585
- PMCID: PMC3653144
- DOI: 10.1038/srep01838
A novel vitreous substitute of using a foldable capsular vitreous body injected with polyvinylalcohol hydrogel
Abstract
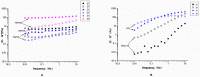
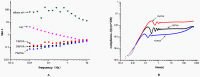
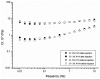
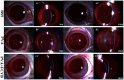
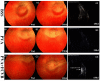
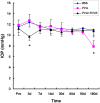
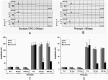
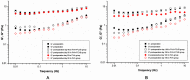
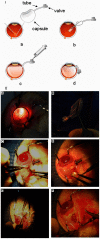
Hydrogels may be the ideal vitreous substitutes due to their wonderful physical features and biocompatibility. However, their drawbacks, short residence time, and biodegradation in vivo, have led to the fact that none of them have been approved for clinical use. In this study, we developed a novel approach of using a foldable capsular vitreous body (FCVB) injected with polyvinylalcohol (PVA) hydrogel as a vitreous substitute for long-term tamponade. The 3% PVA hydrogel that was cross-linked by gamma irradiation showed good rheological and physical properties and had no toxicity in vitro. After 180 days retention, the 3% PVA hydrogel inside FCVB remained transparent and showed good viscoelasticity without biodegradation and showed good biocompatibility and retina support. This new approach may develop into a valuable tool to improve the stability performance of PVA hydrogel as a vitreous substitute and to extend the application function of FCVB for long-term implantation in vitreous cavity.
Figures


References
-
- Federman J. L. & Schubert H. D. Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology. 95, 870–876 (1988). - PubMed
-
- Valone Jr J. & McCarthy M. Emulsified anterior chamber silicone oil and glaucoma. Ophthalmology. 101, 1908–1912 (1994). - PubMed
-
- Ichhpujani P., Jindal A. & Jay Katz L. Silicone oil induced glaucoma: a review. Graefes Arch Clin Exp Ophthalmol. 247, 1585–1593 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

