A specific and sensitive assay for blood levels of glycated CD59: a novel biomarker for diabetes
- PMID: 23670858
- PMCID: PMC3775575
- DOI: 10.1002/ajh.23478
A specific and sensitive assay for blood levels of glycated CD59: a novel biomarker for diabetes
Abstract
Increasing evidence links the complement system with complications of human diabetes. The complement regulatory protein CD59, an inhibitor of formation of membrane attack complex (MAC), is inhibited by hyperglycemia-induced glycation fostering increased deposition of MAC, a major effector of complement-mediated tissue damage. CD59, an ubiquitous GPI-anchored membrane protein, is shed from cell membranes by phospholipases generating a soluble form present in blood and urine. We established an enzyme-linked immunosorbent assay (ELISA) to measure serum/plasma glycated human CD59 (hCD59) (GCD59) and evaluated its potential as a diabetes biomarker. We used a synthetic peptide strategy to generate (a) a mouse monoclonal antibody to capture hCD59, (b) a rabbit monoclonal antibody to detect GCD59, and (c) a GCD59 surrogate for assay standardization. ELISA conditions were optimized for precision, reproducibility, and clinical sensitivity. The clinical utility of the assay was initially evaluated in 24 subjects with or without diabetes and further validated in a study that included 100 subjects with and 90 subjects without a diagnosis of diabetes. GCD59 (a) was significantly higher in individuals with than in individual without diabetes, (b) was independently associated with HbA1c, and (c) identified individuals with diabetes with high specificity and sensitivity. We report the development and standardization of a novel, sensitive, and specific ELISA for measuring GCD59 in blood. The assay distinguished individuals with diabetes from those without, and showed strong correlation between GCD59 and HbA1c. Because GCD59 likely contributes to the pathogenesis of diabetes complications, measurement of blood levels of GCD59 may be useful in the diagnosis and management of diabetes.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
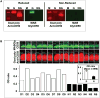
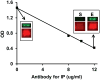

 Represents the pre-formed Nε-glucitollysine residue used to synthesize the (K41(Nε-glucitol)hCD59[37-50] peptide for the position equivalent to K41 in the native protein. The detailed structure of GCD59 synthetic surrogate is shown in Supplemental Figure 3. B. Characteristic surrogate GCD59 standard peptide dose-response curve in the here described sandwich ELISA that utilizes MABTotCD59 as capture and MABGlyCD59 as detection antibodies.
Represents the pre-formed Nε-glucitollysine residue used to synthesize the (K41(Nε-glucitol)hCD59[37-50] peptide for the position equivalent to K41 in the native protein. The detailed structure of GCD59 synthetic surrogate is shown in Supplemental Figure 3. B. Characteristic surrogate GCD59 standard peptide dose-response curve in the here described sandwich ELISA that utilizes MABTotCD59 as capture and MABGlyCD59 as detection antibodies.
Comment in
-
Hematology and diabetes: from hemoglobin A1c to CD59 glycation.Am J Hematol. 2013 Aug;88(8):633. doi: 10.1002/ajh.23500. Epub 2013 Jul 3. Am J Hematol. 2013. PMID: 23757192 Review. No abstract available.
Similar articles
-
Plasma glycated CD59 (gCD59), a novel biomarker for the diagnosis, management and follow up of women with Gestational Diabetes (GDM) - protocol for prospective cohort study.BMC Pregnancy Childbirth. 2020 Jul 18;20(1):412. doi: 10.1186/s12884-020-03090-9. BMC Pregnancy Childbirth. 2020. PMID: 32682411 Free PMC article.
-
Glycation of the complement regulatory protein CD59 is a novel biomarker for glucose handling in humans.J Clin Endocrinol Metab. 2014 Jun;99(6):E999-E1006. doi: 10.1210/jc.2013-4232. Epub 2014 Mar 14. J Clin Endocrinol Metab. 2014. PMID: 24628556 Free PMC article. Clinical Trial.
-
Glycated CD59 is a potential biomarker for gestational diabetes mellitus.Front Endocrinol (Lausanne). 2024 Sep 16;15:1374253. doi: 10.3389/fendo.2024.1374253. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39351537 Free PMC article.
-
Hematology and diabetes: from hemoglobin A1c to CD59 glycation.Am J Hematol. 2013 Aug;88(8):633. doi: 10.1002/ajh.23500. Epub 2013 Jul 3. Am J Hematol. 2013. PMID: 23757192 Review. No abstract available.
-
Role of complement and complement regulatory proteins in the complications of diabetes.Endocr Rev. 2015 Jun;36(3):272-88. doi: 10.1210/er.2014-1099. Epub 2015 Apr 10. Endocr Rev. 2015. PMID: 25859860 Free PMC article. Review.
Cited by
-
Proteomic Profiling of Endothelial Cell Secretomes After Exposure to Calciprotein Particles Reveals Downregulation of Basement Membrane Assembly and Increased Release of Soluble CD59.Int J Mol Sci. 2024 Oct 23;25(21):11382. doi: 10.3390/ijms252111382. Int J Mol Sci. 2024. PMID: 39518935 Free PMC article.
-
Deficiency of the complement regulatory protein CD59 accelerates the development of diabetes-induced atherosclerosis in mice.J Diabetes Complications. 2017 Feb;31(2):311-317. doi: 10.1016/j.jdiacomp.2016.08.021. Epub 2016 Aug 28. J Diabetes Complications. 2017. PMID: 27729184 Free PMC article.
-
Plasma glycated CD59 (gCD59), a novel biomarker for the diagnosis, management and follow up of women with Gestational Diabetes (GDM) - protocol for prospective cohort study.BMC Pregnancy Childbirth. 2020 Jul 18;20(1):412. doi: 10.1186/s12884-020-03090-9. BMC Pregnancy Childbirth. 2020. PMID: 32682411 Free PMC article.
-
Molecular pathogenesis of human CD59 deficiency.Neurol Genet. 2018 Oct 26;4(6):e280. doi: 10.1212/NXG.0000000000000280. eCollection 2018 Dec. Neurol Genet. 2018. PMID: 30533526 Free PMC article.
-
Rapid Non-Enzymatic Glycation of the Insulin Receptor under Hyperglycemic Conditions Inhibits Insulin Binding In Vitro: Implications for Insulin Resistance.Int J Mol Sci. 2017 Dec 2;18(12):2602. doi: 10.3390/ijms18122602. Int J Mol Sci. 2017. PMID: 29207492 Free PMC article.
References
-
- Rosoklija GB, Dwork AJ, Younger DS, et al. Local activation of the complement system in endoneurial microvessels of diabetic neuropathy. Acta Neuropathol (Berl) 2000;99:55–62. - PubMed
-
- Zhang J, Gerhardinger C, Lorenzi M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes. 2002;51:3499–3504. - PubMed
-
- Flyvbjerg A. Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily. Nat Rev Endocrinol. 2010;6:94–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
