18F-fluorobenzoate-labeled cystine knot peptides for PET imaging of integrin αvβ6
- PMID: 23670900
- PMCID: PMC4130341
- DOI: 10.2967/jnumed.112.110759
18F-fluorobenzoate-labeled cystine knot peptides for PET imaging of integrin αvβ6
Abstract
Integrin αvβ6 is a cell surface receptor minimally expressed by healthy tissue but elevated in lung, colon, skin, ovarian, cervical, and pancreatic cancers. A molecular PET agent for integrin αvβ6 could provide significant clinical utility by facilitating both cancer staging and treatment monitoring to more rapidly identify an effective therapeutic approach.
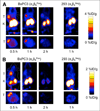
Methods: Here, we evaluated 2 cystine knot peptides, R01 and S02, previously engineered with a 3-6 nM affinity for integrin αvβ6, for (18)F radiolabeling and PET imaging of BxPC3 pancreatic adenocarcinoma xenografts in mice. Cystine knot peptides were labeled with N-succinimidyl-4-(18)F-fluorobenzoate and evaluated for binding affinity and serum stability. Peptides conjugated with (18)F-fluorobenzoate (2-3 MBq) were injected via the tail vein into nude mice xenografted with BxPC3 (integrin αvβ6-positive) or 293 (integrin αvβ6-negative) tumors. Small-animal PET scans were acquired at 0.5, 1, and 2 h after injection. Ex vivo γ-counting of dissected tissues was performed at 0.5 and 2 h.
Results: (18)F-fluorobenzoate peptides were produced in 93% ((18)F-fluorobenzoate-R01) and 99% ((18)F-fluorobenzoate-S02) purity. (18)F-fluorobenzoate-R01 and (18)F-fluorobenzoate-S02 had affinities of 1.1 ± 0.2 and 0.7 ± 0.4 nM, respectively, and were 87% and 94%, respectively, stable in human serum at 37°C for 2 h. (18)F-fluorobenzoate-R01 and (18)F-fluorobenzoate-S02 exhibited 2.3 ± 0.6 and 1.3 ± 0.4 percentage injected dose per gram (%ID/g), respectively, in BxPC3 xenografted tumors at 0.5 h (n = 4-5). Target specificity was confirmed by low tumor uptake in integrin αvβ6-negative 293 tumors (1.4 ± 0.6 and 0.5 ± 0.2 %ID/g, respectively, for (18)F-fluorobenzoate-R01 and (18)F-fluorobenzoate-S02; both P < 0.05; n = 3-4) and low muscle uptake (3.1 ± 1.0 and 2.7 ± 0.4 tumor to muscle for (18)F-fluorobenzoate-R01 and (18)F-fluorobenzoate-S02, respectively). Small-animal PET data were corroborated by ex vivo γ-counting of dissected tissues, which demonstrated low uptake in nontarget tissues with only modest kidney uptake (9.2 ± 3.3 and 1.9 ± 1.2 %ID/g, respectively, at 2 h for (18)F-fluorobenzoate-R01 and (18)F-fluorobenzoate-S02; n = 8). Uptake in healthy pancreas was low (0.3% ± 0.1% for (18)F-fluorobenzoate-R01 and 0.03% ± 0.01% for (18)F-fluorobenzoate-S02; n = 8).
Conclusion: These cystine knot peptide tracers, in particular (18)F-fluorobenzoate-R01, show translational promise for molecular imaging of integrin αvβ6 overexpression in pancreatic and other cancers.
Keywords: cystine knot; integrin αvβ6; positron emission tomography.
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures





Similar articles
-
A Cystine Knot Peptide Targeting Integrin αvβ6 for Photoacoustic and Fluorescence Imaging of Tumors in Living Subjects.J Nucl Med. 2016 Oct;57(10):1629-1634. doi: 10.2967/jnumed.115.169383. Epub 2016 May 26. J Nucl Med. 2016. PMID: 27230926 Free PMC article.
-
99mTc-labeled cystine knot peptide targeting integrin αvβ6 for tumor SPECT imaging.Mol Pharm. 2014 Apr 7;11(4):1208-17. doi: 10.1021/mp400683q. Epub 2014 Feb 24. Mol Pharm. 2014. PMID: 24524409 Free PMC article.
-
68Ga-Labeled Cystine Knot Peptide Targeting Integrin αvβ6 for Lung Cancer PET Imaging.Mol Pharm. 2022 Jul 4;19(7):2620-2628. doi: 10.1021/acs.molpharmaceut.2c00313. Epub 2022 Jun 8. Mol Pharm. 2022. PMID: 35674464
-
64Cu-1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid-Ser-rich Cys knot scaffold grafted with integrin αvβ6-binding peptide RSLARTDLDHLRGR.2012 Aug 16 [updated 2012 Nov 22]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Aug 16 [updated 2012 Nov 22]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23193623 Free Books & Documents. Review.
-
64Cu-1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid-Arg-rich Cys knot scaffold grafted with integrin αvβ6-binding peptide RSLARTDLDHLRGR.2012 Aug 16 [updated 2012 Nov 29]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Aug 16 [updated 2012 Nov 29]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23193614 Free Books & Documents. Review.
Cited by
-
Selection of optimal molecular targets for tumor-specific imaging in pancreatic ductal adenocarcinoma.Oncotarget. 2017 May 26;8(34):56816-56828. doi: 10.18632/oncotarget.18232. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915633 Free PMC article.
-
Druggability of Targets for Diagnostic Radiopharmaceuticals.ACS Pharmacol Transl Sci. 2023 Jul 12;6(8):1107-1119. doi: 10.1021/acsptsci.3c00081. eCollection 2023 Aug 11. ACS Pharmacol Transl Sci. 2023. PMID: 37588760 Free PMC article. Review.
-
A Cystine Knot Peptide Targeting Integrin αvβ6 for Photoacoustic and Fluorescence Imaging of Tumors in Living Subjects.J Nucl Med. 2016 Oct;57(10):1629-1634. doi: 10.2967/jnumed.115.169383. Epub 2016 May 26. J Nucl Med. 2016. PMID: 27230926 Free PMC article.
-
PET/CT Imaging of NSCLC with a αvβ6 Integrin-Targeting Peptide.Mol Imaging Biol. 2019 Oct;21(5):973-983. doi: 10.1007/s11307-018-1296-6. Mol Imaging Biol. 2019. PMID: 30671741
-
Review and Application of Integrin Alpha v Beta 6 in the Diagnosis and Treatment of Cholangiocarcinoma and Pancreatic Ductal Adenocarcinoma.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231189399. doi: 10.1177/15330338231189399. Technol Cancer Res Treat. 2023. PMID: 37525872 Free PMC article. Review.
References
-
- Breuss JM, Gallo J, DeLisser HM, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–2251. - PubMed
-
- Sipos B, Hahn D, Carceller A, et al. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology. 2004;45:226–236. - PubMed
-
- Maubant S, Cruet-Hennequart S, Dutoit S, et al. Expression of alpha V-associated integrin beta subunits in epithelial ovarian cancer and its relation to prognosis in patients treated with platinum-based regimens. J Mol Histol. 2005;36:119–129. - PubMed
-
- Orimoto AM, Neto CF, Pimentel ERA, et al. High numbers of human skin cancers express MMP2 and several integrin genes. J Cutan Pathol. 2008;35:285–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
